Increased interleukin-6 and TP53 levels in rotator cuff tendon repair patients with hypercholesterolemia
Article information
Abstract
Background
A previous study reported that hyperlipidemia increases the incidence of tears in the rotator cuff tendon and affects healing after repair. The aim of our study was to compare the gene and protein expression of torn rotator cuff tendons in patients both with and without hypercholesterolemia.
Methods
Thirty patients who provided rotator cuff tendon samples were classified into either a non-hypercholesterolemia group (n=19, serum total cholesterol [TC] <200 mg/dL) and hypercholesterolemia group (n=11, serum TC ≥240 mg/dL) based on their concentrations of serum TC. The expression of various genes of interest, including COL1A1, IGF1, IL-6, MMP2, MMP3, MMP9, MMP13, TNMD, and TP53, was analyzed by real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR). In addition, Western blot analysis was performed on the proteins encoded by interleukin (IL)-6 and TP53 that showed significantly different expression levels in real-time qRT-PCR.
Results
Except for IGF1, the gene expression levels of IL-6, MMP2, MMP9, and TP53 were significantly higher in the hypercholesterolemic group than in the non-hypercholesterolemia group. Western blot analysis confirmed significantly higher protein levels of IL-6 and TP53 in the hypercholesterolemic group (p<0.05).
Conclusions
We observed an increase in inflammatory cytokine and matrix metalloproteinase (MMP) levels in hypercholesterolemic patients with rotator cuff tears. Increased levels of IL-6 and TP53 were observed at both the mRNA and protein levels. We suggest that the overexpression of IL-6 and TP53 may be a specific feature in rotator cuff disease patients with hypercholesterolemia.
INTRODUCTION
Rotator cuff repair is widely practiced as a treatment method for rotator cuff tears. However, failure of the rotator cuff to heal after surgical treatment is a well-known complication that is reported in 20%‒94% of cases [1]. Fatty degeneration is an important prognostic factor that determines the anatomical and functional outcome after rotator cuff repair [2]. However, it is difficult to reverse the progress of fatty degeneration by rotator cuff repair alone [2].
Hypercholesterolemia is a crucial health problem that is associated not only with heart disease but also with tendon pathology [3]. Lipid-related changes in tendon pathology affect several mechanical properties of the tendon, including stiffness and modulus [4]. Multiple mechanisms have been proposed to clear these cholesterol-related changes, including alterations in tenocyte protein and gene expression, matrix turnover, cytokine production, and tissue vascularity [5]. A previous study reported that hyperlipidemia increases the incidence of tears in the rotator cuff tendon and affects healing after repair [6]. In animal models, hypercholesterolemia has been found to cause a decrease in the biomechanical properties of the tendon-to-bone healing of the rotator cuff [7]. However, few studies have reported differences in molecular level changes on the effects of hypercholesterolemia in rotator cuff tears.
The rotator cuff healing process is divided into three stages: inflammation, repair, and remodeling. This healing process is accomplished by various molecular mediators. The healing process of the tendon is initially composed of collagen type III, which is replaced by collagen type I, thus increasing the collagen type-I-to-III ratio [8]. Collagen type I is encoded by the COL1A1 and COL1A2 genes, respectively. In an in vitro study, tendon cells were shown to synthesize only collagen type I [9]. A different in vitro study found that insulin-like growth factor-1 (IGF-1) increased collagen synthesis in tendons and ligaments by stimulating fibroblast proliferation and synthesis of extracellular matrix (ECM) proteins [10]. In addition, it has been demonstrated that IGF-1 promotes the healing of tendons and ligaments in animals [11]. Interleukin (IL)-6 is one of the cytokines involved in triggering the inflammatory cascade in the early phase of the tendon healing process [12]. Moreover, it leads to collagen production in tendons and is significantly elevated after both exercise and trauma [13]. Matrix metalloproteinases (MMPs) are believed to play an important role in ECM remodeling during the remodeling phase of tendon healing [14]. MMP2, MMP9, and MMP13 are involved in cell transformation and morphogenesis as well as degradation in both pathological and non-pathological conditions [15]. Tenomodulin (TNMD) has been confirmed to be a relatively specific molecular marker of late tendon differentiation and plays a central role in the development and maturation of tendons [16,17]. p53 is a tumor suppressor protein known to inhibit fatty acid synthesis and lipid accumulation and to promote programmed cell death of tendon cells in rotator cuff tendinopathy [18-20].
In the present study, the gene expression levels of nine molecular mediators were analyzed in the rotator cuff tendon of patients both with and without hypercholesterolemia. The protein expression levels of the molecular mediators that showed significant differences in gene expression levels were analyzed. We hypothesized that hypercholesterolemia would affect the gene and protein expression of molecular mediators involved in tendon healing in torn rotator cuff tendons. Understanding the molecular basis of lipid-related changes in rotator cuff tendons may eventually prevent the progression of these changes and improve outcomes after rotator cuff repair.
METHODS
This study was approved by the Institutional Review Board of Kyungpook National University (No. KNUH 2016-11-020) including the procedure for informed consent from participants based on the Declaration of Helsinki in the study of human participants.
Participants
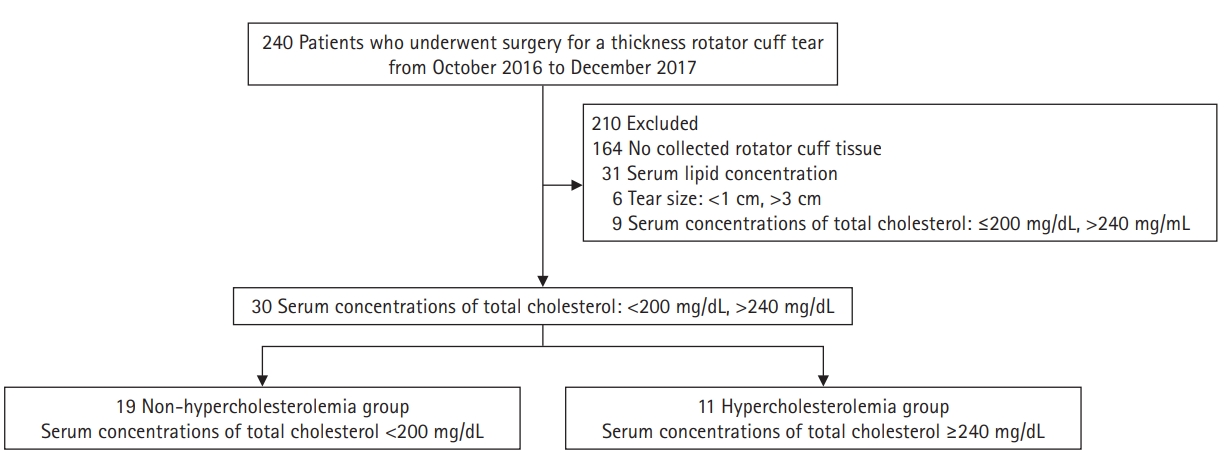
From October 2016 to November 2017, 240 patients who underwent arthroscopic rotator cuff repair for a full-thickness rotator cuff tear at our institution were enrolled in this study. Among them, 164 patients who could not contribute tissue from the rotator cuff tendon without prior informed consent were excluded. Among 76 patients, patients without preoperative serum lipid evaluation (n=31) and with anteroposterior dimension of tear size <1 cm or >3 cm (n=6) were excluded. Finally, patients with a borderline serum total cholesterol (TC) of ≥200 mg/dL and ≤240 mg/dL (n=9) were excluded from the diagnostic criteria for hyperlipidemia [21]. Thirty patients were classified into either the non-hypercholesterolemia group (n=19, TC <200 mg/dL) or the hypercholesterolemia group (n=11, TC ≥240 mg/dL) based on the concentrations of TC (Fig. 1). In the preoperative magnetic resonance imaging, any fatty infiltration of the supraspinatus, infraspinatus, and subscapularis muscles was graded according to the classification system of Goutallier et al. [22].
Tendon Tissue Collection from Patients
All patients included in the study provided informed consent for tissue collection of residual rotator cuff tendons that occurred during the debridement process during surgery. Specimens of about 5 mm× 5 mm were obtained from the tendons, placed in labeled plastic tubes with RNAlater (QIAGEN, Valencia, CA, USA) for nucleic acid extraction, and then transferred to a –80°C freezer until processing.
RNA Extraction and cDNA Synthesis
Frozen tissue samples stored at –80°C were homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA, USA) using an OMNI TH Homogenizer (OMNI International, Kennesaw, GA, USA). RNA extraction was carried out as per the manufacturer’s protocol using TRIzol reagent (Invitrogen). The RNA concentration and quality were determined by measuring the ratio of absorbance at 260 nm to that at 280 nm using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), with all samples achieving a minimum ratio of 1.80. The RNA (250 ng) was reverse-transcribed using an iScript Reverse Transcription Supermix for quantitative reverse transcription polymerase chain reaction (qRT-PCR; Bio-Rad, Hercules, CA, USA).
Gene Expression by Quantitative Real-Time PCR
Complementary DNA was diluted to 2.5 ng/μL with Rnase-free water, and 5 μL of this solution was used to run a 20-μL quantitative polymerase chain reaction with iTaq Universal Probes Supermix (Bio-Rad). Validated human primers included GAPDH (ID: qHsaCEP0041396), COL1A1 (ID: qHsaCEP0050510), IGF1 (ID: qHsaCEP0041360), IL-6 (ID: qHsaCEP0051939), MMP2 (ID: qHsaCEP0049822), MMP3 (ID: qHsaCIP0026053), MMP9 (ID: qHsaCIP0028098), MMP13 (ID: qHsaCIP0026824), TNMD (ID: qHsaCIP0029219), and TP53 (ID: qHsaCEP0052284) (Bio-Rad). Duplicate reactions for each gene were run on a CFX96 touch real-time PCR machine (Bio-Rad), and the mean value for these duplicates was calculated and used for the analysis. Amplification reactions were performed with 40 cycles (95°C for 2 minutes, 95°C for 5 seconds, and 60°C for 30 seconds), and the results were normalized to GAPDH expression and calculated using CFX Manager 3.1 software (Bio-Rad).
Western Blot Analysis
Proteins were detected with the following antibodies and reagents. Total proteins were extracted using a radioimmunoprecipitation assay lysis buffer (Rockland Inc., Limerick, PA, USA) containing a protease inhibitor cocktail (Quartett, Berlin, Germany). The total proteins (20 μg/sample) were applied to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the proteins were transferred to nitrocellulose membranes using the Trans-Blot Turbo Transfer System (Bio-Rad). The membranes were blocked with tris-buffered saline containing 5% skim milk and 0.2% Tween 20. Primary antibodies were used against the following proteins: IL-6 (Abcam, Cambridge, MA, USA), p53 (Cell Signaling Technology, Danver, MA, USA), and GAPDH (Cell Signaling). After reaction with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA), the protein bands on the membranes were visualized using a Clarity Western ECL Substrate Chemiluminescence Assay Kit (Bio-Rad) following the manufacturer’s suggested procedure. Densitometry of the bands was performed using a Chemi-Doc XRS+ Imaging System (Bio-Rad) and normalized to GAPDH band intensity.
Statistical Analyses
The mean values were compared using the Student t-test or Mann-Whitney U-test for continuous variables and the chi-square or Fisher’s exact test for categorical variables to statistically evaluate the differences between groups. The statistical analysis was conducted using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA) with the significance level set at p<0.05. The data are presented as the mean±standard deviation. A post hoc power analysis was performed on 30 patients, and the true effect size was evaluated using an α of 0.05 and an average effect of 0.8. In order to derive a significant result, the sample was analyzed as having 66% power.
RESULTS
Demographic Data
According to the demographic and clinical data, age, sex, prevalence of hypertension, diabetes and hyperthyroidism, rotator cuff tear size, fatty infiltration, duration of symptoms, and visual analog scale score were not significantly different between the two groups. The hypercholesterolemia group had higher serum TC and low-density lipoproteins concentrations (246.27±7.79 mg/dL and 157.45±21.93 mg/dL) as compared with the non-hypercholesterolemia group (192.87±16.22 mg/dL and 116.72±28.44 mg/dL) (p=0.009 and p=0.009, respectively). Serum high-density lipoprotein concentrations in the non-hypercholesterolemia group (66.20±12.99 mg/dL) were significantly higher than in the hypercholesterolemia group (43.36±9.08 mg/dL) (p=0.012). Serum triglyceride concentrations were not significantly different between the two groups (p=0.108) (Table 1).
Gene Expression by Quantitative Real-Time PCR
Among the cytokines, IL-6 mRNA levels were the highest (mean, 10.90±6.71), and the mRNA levels of MMP2 (mean, 4.98±3.33), MMP9 (mean, 2.03±1.56), and TP53 (mean, 8.97±5.79) were also significantly higher in the hypercholesterolemia group. In contrast, only IGF1 mRNA levels (mean, 8.87±5.87) were significantly higher in the non-hypercholesterolemia group (Table 2). These results indicated that hypercholesterolemia could influence the inflammatory response in rotator cuff tendon tissue (p<0.05).
Western Blot
To investigate the effect of hypercholesterolemia on protein expression, an immunoblotting analysis was performed with antibodies against IL-6 and p53 based on the results of qRT-PCR; GAPDH was used as the loading control. A comparison of the Western blot band intensities (mean, 0.46±0.24 and 0.23±0.19, respectively) for IL-6 and TP53 revealed that their protein levels were significantly higher in patients with hypercholesterolemia (Table 3).
DISCUSSION
In this study, we found that in torn rotator cuff patients, the gene expression levels of IL-6, MMP2, MMP9, and TP53 were significantly higher in patients with hypercholesterolemia compared to those without hypercholesterolemia, and the gene expression of IGF1 was significantly higher in patients without hypercholesterolemia. Upon Western blot analysis, the expression of IL-6 and TP 53 proteins was significantly higher in patients with hypercholesterolemia than in those without.
The incidence of hypercholesterolemia is rapidly increasing in the elderly population and manifests as a debilitating medical condition accompanied by numerous systemic complications. In a high-cholesterol environment, lipids accumulate within the tendon ECM, forming a precipitate called a “yellow species.” These lipid-related changes affect a variety of mechanical properties, including modulus and stiffness, in intact tendons [4]. There are several mechanisms that explicate these cholesterol-related changes, including changes in the tenocyte protein and gene expression, matrix turnover, cytokine production, and tissue vascularity. Hypercholesterolemia can alter the ECM of the tendons so that the damage is increased or becomes difficult to heal [5].
A previous study reported that hyperlipidemia increases the incidence of tears in the rotator cuff tendon and affects healing after repair [6]. However, the effects of hypercholesterolemia on the tendon at the molecular level are not yet known. In this study, we found significant overexpression of IL-6 and TP53 in the torn rotator cuff tendons of patients with hypercholesterolemia when compared with those of controls. IL-6 is a cytokine involved in the regulation of the immune response and inflammation or hematopoiesis, and it acts on various cells [12]. Cytokines can influence a wide array of ECM components [23]. In addition, IL-6 has been shown to be responsible for the inhibitory effects of wound fluid on fibroblast division [24]. Moreover, it leads to collagen production in tendons and is significantly elevated after both exercise and trauma [13]. TP53 dominates the cell cycle, induces cell death, and plays an important role in tumor suppression through its regulation of protein-related metabolism. In addition, previous studies have shown that TP53 regulates lipid metabolism by direct protein-protein interactions or transcriptional control of the proteins involved in fatty acid synthesis, fatty acid oxidation, the mevalonate pathway, lipid droplet formation, and cholesterol efflux [18]. Generally, TP-53 suppresses fatty acid synthesis and lipid accumulation.
No studies have been conducted on the changes in TP53 levels in hypercholesterolemia or its effect on the rotator cuff tendon healing process. In their study of different types of organs, Yao et al. [25] confirmed an increase in p53 levels in the kidneys of mice with hypercholesterolemia and reported that p53 induced apoptosis in the kidneys. A previous study reported a significant increase in p53 levels in supraspinatus tears and speculated that tenocyte apoptosis may be a relatively early feature in rotator cuff tendinopathy [20]. Kane and Greenhalgh [26] found that wounds in diabetic animals displayed a delayed onset of p53 transcription but had persistently greater levels for longer periods of time. Diabetic animals appear to lose the indirect relationship between p53 and bcl-2. These findings suggest that p53 levels are increased in the early phase of healing, after which it becomes necessary to stop the inflammatory process and decrease p53 levels to allow cell proliferation to occur for tissue repair. In patients with hypercholesterolemia, fatty acid synthesis and lipid accumulation in the rotator cuff tendon are increased, which maintains the expression of TP53 in an elevated state for an extended time and may affect rotator cuff healing. Abboud and Kim [6] reported that patients with rotator cuff tears were more likely to have hypercholesterolemia than were those without tears. Chung et al. [3] observed that high cholesterol levels had a significant effect on rotator cuff healing in a rat model. To some extent, controlling hypercholesterolemia could stop or reverse the harmful effects of hypercholesterolemia even after rotator cuff canine repair surgery in a rat model. Despite these findings from these different studies, the pathophysiology of lipid-related tendon pathology remains incompletely understood [27].
In our study, IL-6 and TP53 levels were significantly higher in hypercholesterolemic patients who had undergone a rotator cuff repair. However, little is known about the effects of hyperlipidemia on the rotator cuff tendon at the molecular level. Several studies have reported the effects of lipid-lowering agents on cytokine levels in different tissues. Researchers who investigated the effects of cholesterol synthesis inhibitors on cytokine production capacity in vitro have explained the inhibitory effects on the production of several cytokines. Lovastatin inhibits lipopolysaccharide-induced synthesis of proinflammatory cytokines, such as tumor necrosis factor-α, IL-1βα, and IL-6, in rat primary astrocytes, microglia, and macrophages [28]. Sakoda et al. [29] reported that simvastatin reduces IL-1α-induced production of inflammatory cytokines, such as IL-6 and IL-8, in human oral epithelial cells. Thus, simvastatin has an anti-inflammatory effect on human oral epithelial cells via mechanisms that are independent of cholesterol lowering. The effects of statins on cytokine levels in other tissues in hypercholesterolemia remain unclear, which is also the case for the rotator cuff tendon.
This study had some limitations to consider for further study. First, although IL-6 and TP53 levels were significantly higher in patients with hypercholesterolemia, there was still insufficient evidence for the association of IL-6 and TP53 with hypercholesterolemia in this study. In addition, the protein expression of all molecular mediators that showed significant differences in gene expression have not yet been analyzed. Second, although it is known that hypercholesterolemia affects various mechanical properties of the tendon, it is still unclear whether elevations in IL-6 and TP53 expression have any significant effect on the healing of the rotator cuff in the presence of hypercholesterolemia. Third, the present study only analyzed the expression of genes and proteins in tissues either with or without hypercholesterolemia. Thus, we did not consider any other comorbidity that might affect the expression of these genes and proteins. Chung et al. [30] demonstrated that overexpression of MMP-9 and IL-6 may be one of the causes of high healing failure rates after rotator cuff repair in diabetic patients. Fourth, Tucker and Soslowsky [31] showed that treatment with simvastatin for 3 months alters some mechanical and histological properties of the tendon in a model of diet-induced hypercholesterolemia. Their simvastatin group had significantly more spindle-shaped cells in the midsubstance region of the supraspinatus muscle than their hypercholesterolemia group. Additionally, these data suggest that simvastatin use does not have any strong negative effect on the mechanical and histological properties of tendons, which implies that patients prescribed simvastatin may not experience any tendon damage. Among patients with hypercholesterolemia, those who were taking medication for treatment were not excluded from the study. Therefore, drug-induced changes in cytokine and growth factor production were not reflected in the results. Garcia et al. [32] reported that hypercholesterolemia was a significant risk factor for re-tears after arthroscopic rotator cuff repair. However, the type and dose of statin medication did not significantly affect the incidence of re-tears. Fifth, we could not include all the cytokines or growth factors relevant to tendon tears or hypercholesterolemia. Instead, we evaluated only selected cytokines or growth factors that were of our interest. Including more cytokines or growth factors in the analysis could detect other factors that may be related to rotator cuff tears in patients with hypercholesterolemia.
Our results showed an increase in inflammatory cytokine and MMP levels in tendon tissues obtained from patients with hypercholesterolemia who had undergone rotator cuff repair. Significantly higher IL-6 and TP53 levels were observed in the torn cuff tendon tissues not only at the mRNA level but also at the protein level. We suggest that the overexpression of IL-6 and TP53 may be an important feature in rotator cuff tears in patients with hypercholesterolemia.
Notes
Financial support
This work was supported by Biomedical Research Institute grant, Kyungpook National University Hospital (2021).
Conflict of interest
Jong Pil Yoon is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.