Assessment of therapeutic clinical trials for proximal humeral fractures
Article information
Abstract
Proximal humeral fractures (PHFs) are a common injury among the older population. An ideal therapeutic protocol has yet to be developed, and numerous clinical trials are being conducted to find the best therapeutic approach. The purpose of this study is to evaluate the current body of knowledge available via interventional clinical trials.In December 2022, interventional clinical trials relating to PHFs on Clinicaltrials.gov were screened. Trial characteristics included duration, status, intervention, phase, outcomes, location, and study design. Publications associated with each trial were searched on PubMed/Medline using the ClinicalTrials.gov registry number.The final dataset comprised 64 trials. The most common trial status was completed (36%). The majority did not have a Food and Drug Administration-defined phase (67%), was randomized (81%), involved a single facility (72%), used a parallel assignment intervention model (80%), and used an open-label approach (45%). Eleven trials were associated with a publication, and the publication rate was 17%. Average enrollment was 86 participants, and mean trial duration was 51.4 months. Europe/UK/Russia/Turkey participated in the most trials (70%). Most of the trials were initiated after 2010 (87.5%). Procedure-related interventions (55%) were most common. Disability/function was the most common primary outcome assessed (61%). The low publication rate and the multitude of trials conducted after 2010 highlight the urgency and need for trial results to be published to establish an ideal therapeutic protocol. Since the majority of the trials involved a single institution and an open-label approach, reinforcing blinding and establishing multi-centered trials can improve the validity of the clinical trial results.
INTRODUCTION
Proximal humeral fractures (PHFs), which make up around 5% of all fractures, are frequent injuries in the older population [1]. PHFs are the third most often observed fragility fracture in the last 20 years, making them a significant cause of morbidity in many osteoporotic patients in our aging population [2]. PHFs are most frequently caused by a low-energy fall in an osteoporotic patient, leading to primarily non-displaced two-part fractures that are frequently treated conservatively with immobilization in a sling with positive clinical outcomes [3].
The type of fracture and the patient characteristics must be taken into account while deciding whether to perform surgery. The majority of PHFs may not require surgery. Recent research does, however, support surgical intervention in displaced three- and four-part fracture patients [4]. The surgeon must choose the device to use for operative intervention, which can be challenging, even for skilled surgeons. LaMartina et al. [5] pointed out the difficulty in choosing a course of treatment, finding that fellowship-trained shoulder surgeons agreed just 63.5% of the time. In the past, hemi-arthroplasty (HA) was used to repair these fractures in older patients and open reduction internal fixation (ORIF) was used in younger patients [6,7]. Reverse shoulder arthroplasty (RSA), however, is becoming more common [8,9]. While the use of HA has significantly decreased over time and the use of RSA has dramatically increased, the use of ORIF has remained consistent [10]. According to Acevedo et al. [11], RSA may be especially helpful for patients older than 70 years. A patient's health situation and the type of fracture may also be taken into consideration in addition to age when making decisions [12,13].
Despite this, we have not yet developed an ideal therapeutic protocol for treatment of this condition, and numerous clinical trials are being conducted to find the best therapeutic approach to treat PHF. Clinical trial evaluation and assessment are crucial for identifying successful tactics and treatments, as well as for identifying gaps and prospective areas for improvement. The purpose of this study is to evaluate the current body of knowledge available for PHF via interventional clinical trials.
METHODS
Search Strategy and Selection Criteria
ClinicalTrials.gov—a database registering clinical studies worldwide that are privately and publicly funded on a weekly basis—was screened for information regarding interventional clinical trials targeting PHFs [14]. For the clinical trials to be included in the database, details such as a description of the protocol, inclusion/exclusion criteria, outcome measures, and any relevant history must be provided by the investigator of the respective trial. Previous literature has utilized clinical trial data from this database to reach conclusions [15,16]. Institutional Review Board approval was not required for this study.
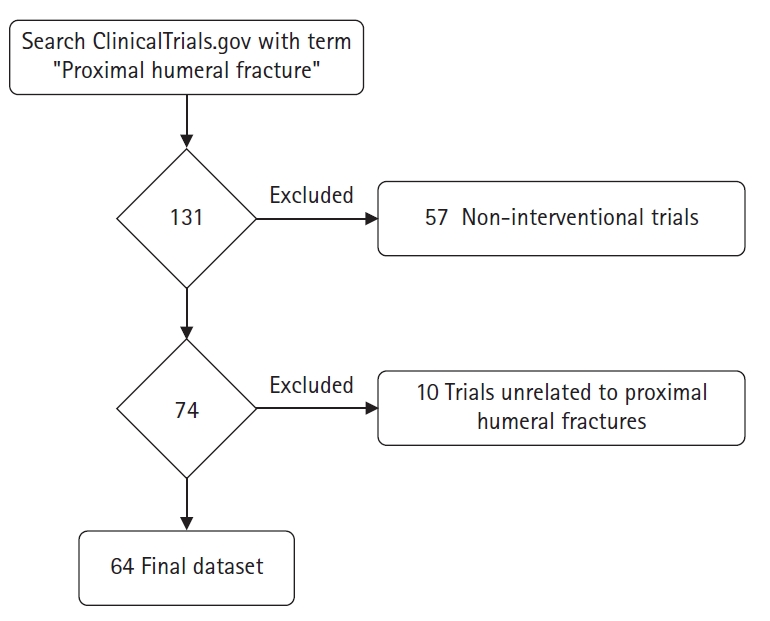
In December 2022, the ClinicalTrials.gov registry was queried using the key words “Proximal humeral fracture” without any search restrictions to find all related clinical trials. A total of 131 relevant trials was generated from the search. Similar to studies in previous literature, non-interventional trials were excluded, eliminating 57 trials [15,16]. An additional 10 trials were removed from the study as they did not focus on PHFs. Ultimately, 64 clinical trials remained in the final dataset (Fig. 1).
Data Collection
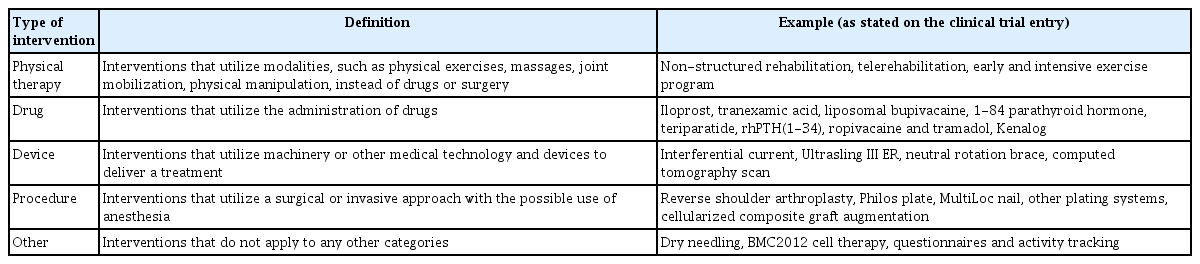
Information collected from the final set of clinical trials included the status (not yet recruiting, active-not recruiting, enrolling by invitation, recruiting, completed, terminated, withdrawn, and unknown), trial duration, type of experimental intervention, phase, number of testing locations, sample size, primary outcomes, secondary outcomes, study design (intervention model, allocation, and masking), and publications. If the trial was not completed, the estimated end dates listed on the registry were used to determine the duration. If a clinical trial was conducted on multiple continents, then both were associated with the trial in the data collection. The different interventions were categorized into: physical therapy, drug, device, procedure, and other. Examples and definitions of the different intervention types are presented in Table 1.
Review of Literature
PubMed/Medline was used to search for publications associated with each trial by inputting the ClinicalTrials.gov registry number (NCTID). Each of these publications was reviewed, and the primary results from the trials were analyzed using the available publications.
RESULTS
Trial Characteristics
A total of 64 trials was included in our study. The general characteristics of the clinical trials were presented in Table 2 and organized according to trial phase. Most trials did not have an Food and Drug Administration (FDA)-defined phase (not applicable; n=43, 67%). The most common status among the clinical trials was completed (n=23, 36%). On the other hand, only one trial was enrolling by invitation. Fifteen trials were recruiting, seven were terminated, seven had an unknown status, five were active but not recruiting, four were not yet recruiting, and two were withdrawn. Trials were terminated or withdrawn for various reasons: slow recruitment (NCT02362100, NCT01532076, NCT03599336, NCT03017105, NCT02091492), lack of funding (NCT02362100), difficulties maintaining follow-up (NCT02362100), release of a publication indicating that the intervention was not always beneficial (NCT02073695), opened under a new principal investigator (NCT02597972), and other unrelated reasons (NCT00326794, NCT00741182).
The most common estimated enrollment size among the trials was between 51 to 100 patients (n=29, 45%). Average enrollment was 86 participants. Only a few trials were initiated before 2010, with 87.5% beginning after 2010. Trial durations ranged from two months to 204 months with a mean of 51.4 months. Europe/UK/Russia/Turkey participated in the largest number of clinical trials (n=45). This was followed by North America with 11 trials, Asia with six trials, South America with two trials, and Africa/Egypt with one trial.
Types of Interventions
Table 3 presents the distribution of the types of interventions used among the trials in the final dataset. The most common type of intervention was procedure-related interventions, which was studied in 35 (55%) trials. This was followed by drug-related interventions (n=11, 17%), physical therapeutic modalities (n=9, 14%), and device-related interventions (n=5, 8%). Four trials (6%) involved an intervention that did not apply to the other categories.
Primary Endpoints
Of the trials, 46 (72%) involved a single facility, while 11 were multi-centered. Seven of the clinical trials did not report the number of institutions involved in the trial (Table 4). In 52 trials (81%), patients were randomized into groups; three trials were non-randomized. The mode of allocation was not reported for nine trials. The majority of the trials utilized a parallel assignment intervention model (n=51, 80%), while 11 trials involved a single group assignment model, and two trials involved a crossover assignment model. The most common approach was an open-label approach (no masking), in 29 trials (45%). Of all trials, 19 trials single-masked, 12 were double-masked, two were triple-masked, and two were quadruple masked.
The majority of the trials analyzed one primary endpoint; however, four trials explored two primary endpoints. The most common primary endpoint was disability/function, which was used in 39 trials (61%). This was followed by radiographic evaluation with 11 trials, pain with eight trials, adverse events with seven trials, quality of life/patient satisfaction with one trial, and range of motion with one trial. The most common primary outcome assessor was the Constant-Murley Score (21 trials), which was followed by the Disabilities of the Arm, Shoulder, and Hand Score (DASH) or its short version QuickDASH Score (9 trials).
Trial Results and Publications
Results were available for 11 trials, which resulted in a 17% publication rate. Fifteen original publications, excluding publication of a systematic review, were produced from the included trials [17-31]. Three of the trials were linked to multiple original publications. Thirteen of the 15 publications were associated with a trial that was not defined by a phase, one publication was associated with a trial in phase II, and one publication was associated with a trial in phase IV (Table 5). Eight publications were protocols for the respective clinical trial and did not report any results. Three publications compared the outcomes of different surgical interventions. Although two of the three publications found no significant differences between the interventions, one suggested that an RSA was more advantageous than an ORIF at the 2-year follow-up in the treatment of displaced type-B2 and C2 PHFs in elderly patients. Although another publication suggested no significant difference between outcomes in patients who received surgical versus conservative interventions, another publication suggested that surgical intervention resulted in better functional and radiographic outcomes at the 1-year follow-up. Additionally, one publication explored whether a T2-level thoracic paravertebral block was necessary when treating patients undergoing a deltopectoral approach for PHF treatment. Despite the decreased risk of incomplete regional anesthetic, the T2-level thoracic paravertebral block was suboptimal due to the potential risks. Further, one publication explored whether teriparatide could enhance the healing of a PHF and concluded that teriparatide resulted in better healing on radiographic assessment even though there was no significant difference in pain or functional outcomes. Additional details of the publications can be found in Table 5.
A systematic review assessing the benefits and harms of non-pharmaceutical treatments for PHFs in adults was performed by searching the Cochrane Central Register of Controlled Trials, Medline, Embase, trial registries, and bibliographies of trial reports. Systematic reviews to September 2020 included 31 of the 64 trials from this final dataset [3]. The review analyzed mostly women older than 60 years, and most of the trials were at high risk of bias due to a lack of blinding. There was insufficient evidence to draw a conclusion regarding the difference between surgical, non-surgical, or rehabilitation interventions for PHFs.
DISCUSSION
As of December 2022, only 64 therapeutic clinical trials were identified that explored the management of PHFs. The trials spanned 23 countries and five continents, with Europe sponsoring the largest number of trials at 45. North America followed with 11 trials, while South America and Africa had only two and one trials, respectively. Research on shoulder pathologies has been increasing in Europe in recent decades [32,33]. European PHF research, in specific, has produced numerous publications on the epidemiology of the injury in several European countries, as well as management protocols and treatment trends [34-37]. Europe had the highest number of trials in our study, likely due to its impressive research infrastructure and high investment in the management of shoulder pathologies [32-37]. Moreover, the majority of the trials was started after 2010, indicating the novelty of this topic. Management of PHF was originally mostly conservative [38]. However, as years passed, integrating operative management for patients with displaced fractures showed good clinical and functional outcomes [39]. As such, research on PHF treatment increased in prominence as innovative technological advancements led to the development and popularization of new surgical options. Despite that, the publication rate found in our study was relatively low, at 17%. This low rate is problematic as it downplays the efforts put into these clinical trials and reduces the reliability of the collectively reported trial results. Low publication rates constitute a major problem in that regard and are often explained by hesitancy to report negative results, absence of academic expectations or incentives, lack of funds, or language difficulties [15,40].
The majority of trials had appropriate models and designs. Most were randomized and had parallel assignment models, allowing appropriate comparison between treatment modalities. Since the trials mainly involved variations of verified treatments, the majority did not have an FDA-defined phase. In specific, the majority of trials explored procedure-related interventions [14]. Such analysis is valid in these circumstances since the treatment of PHFs mainly relies on fracture characteristics. While conservative treatment can be pursued for minimally displaced fractures, more critical cases require surgical intervention for optimizing function [39,41,42]. Hence, it is important to explore the different surgical options available for this pathology to help further develop treatment guidelines and strategies.
The published results explored anesthesia-related, conservative, and surgical treatment options in the setting of PHFs. In a study by Wang et al. [20], adding a T2-level thoracic paravertebral block was helpful to decrease the occurrence of incomplete anesthesia in elderly patients undergoing a PHF surgery using a deltopectoral approach; however, it does not eliminate all such cases and does involve potential risks. Understanding the benefits and risks of administering regional anesthesia is important to reduce the risk of increased postoperative pain. In a study by Johansson [17], even though conservative treatment with teriparatide injections was correlated with better radiographic outcomes, there was no significant improvement in pain or function.
Regarding surgical options, in a study by Fraser et al. [24], RSA had better clinical results than an ORIF using an angular stable plate at 2-year follow-up for type-C2 PHFs in elderly patients, while there was no significant difference for type-B2 PHFs in elderly patients. In a study by Bønes et al. [25], using pegs instead of screws for fixation of PHFs showed no significant difference in the development of avascular head necrosis. However, the use of pegs and screws both showed better functional outcomes at 12 months when the patient did not have a joint penetration. In a study by Hengg et al. [30], cement augmentation of the proximal humerus internal locking system screws had a slightly higher mechanical failure rate compared to the group without cement augmentation.
The controversy between conservative and surgical treatment was also addressed. In a study by Fjalestad et al. [26], at the 12-month follow-up, even though an ORIF using an angular stable plate and cerclages had significantly better radiographic outcomes compared to nonoperative treatment, there was no significant difference in clinical outcomes. Launonen et al. [27] observed no significant difference between outcomes at 2-year follow-up for patients who received a Philos locking plate, Epoca prosthesis, or conservative treatment (sling) in the setting of a two-part PHF. However, the operative group experienced three cases of complications that required a subsequent surgery. In both studies, no interventional modality was superior.
All but four trials explored one primary endpoint, while the rest explored two endpoints. The most commonly explored endpoint was function/disability, and the most commonly utilized outcome score was the Constant-Murley score. This outcome scoring tool is appropriate in this setting since it helps explore pain, function, range of motion, and strength of the affected shoulder [43,44]. That being said, several study design limitations existed. The majority of trials involved only a single institution, and around 45% adopted an open label approach. This increased the risk of bias and decreased the generalizability and reliability of trial results [45].
CONCLUSIONS
Assessing the interventional clinical trials targeting PHFs can help to establish an ideal therapeutic protocol. The urgency and need to establish an ideal therapeutic protocol can be seen by the multitude of trials conducted after 2010, most of which were procedure related. Although there were numerous trials, the publication rate was low. The common use of a single institution and the common use of an open-label approach need to be addressed in future studies.
Interventions targeting PHFs are mainly categorized into physical therapeutic modalities, drug-related interventions, device-related interventions, procedure-related interventions, and other interventions that do not apply to the other categories. The majority of the trials evaluated procedure-related interventions, which emphasizes the interest in establishing an ideal treatment protocol, as well as advantages and disadvantages of different options. Primary endpoints mainly involved disability/function, radiographic evaluation, pain, and adverse events. Further study is needed to determine the effectiveness and advantages of different treatment options to determine the best protocol.
Notes
Author contributions
Conceptualization: JK, MYF. Data curation: JK, MYF, MD. Formal analysis: JK, MD. Methodology: MYF. Project administration: JK. Supervision: JK, JAA. Validation: JK. Writing – original draft: MYF, MD. Writing – review & editing: JK, MYF, MD, JAA.
Conflict of interest
JAA would like to disclose royalties from: DJO Global, Zimmer-Biomet, Smith and Nephew, Stryker, Globus Medical, Inc.; research support as a PI from: Lima Corporation - Italy, Orthofix, Arthrex, OREF; royalties, financial or material support from: Wolters Kluwer; and board member/committee appointments for: American Shoulder and Elbow Society, Pacira.
Funding
None.
Data availability
None.
Acknowledgments
None.