Surgical management of biconcave glenoids: a scoping review
Article information
Abstract
Biconcave (B2) glenoids, characterized by significant posterior glenoid bone loss and a biconcave wear pattern, are a challenging pathology in shoulder surgery. Significant bone defects present in B2 glenoids increases the risk of complications and rates of failure for operative patients with glenohumeral osteoarthritis. Diagnosing this entity is of pivotal importance, and can be accomplished with imaging and a comprehensive clinical investigation. There are no clear-cut guidelines for management, but options include hemiarthroplasty, anatomic total shoulder arthroplasty, and reverse shoulder arthroplasty. In recent years, modern techniques such as corrective reaming, bone grafts, and the use of augmented components have improved patient outcomes. Educating prospective patients is essential for reaching a shared management decision, setting appropriate expectations, and optimizing prognostic outcomes.
INTRODUCTION
Glenohumeral osteoarthritis is a prevalent degenerative disease that causes substantial changes in the stability and morphology of the shoulder, resulting in a wide range of deformities [1,2]. Several factors are involved in the development of such deformities, including a significant increase in native retroversion of the glenoid, the percentage of glenoid bone loss, the range of depth of the posterior concavity and erosion, and the posterior translation of the humeral head translating into the defect, leading to progressive subluxation [1-4]. In 1999, Walch classified the changes in glenoid morphology that occur during glenohumeral osteoarthritis by assessing posterior glenoid wear and static posterior subluxation of the humeral head [1-4]. A biconcave (B2) glenoid, which is characterized by preservation of the anterior portion of the native glenoid with asymmetric wear of the posterior glenoid, presents a unique challenge for shoulder surgeons worldwide due to the poor bony foundation of the B2 glenoid, along with high failure and complication rates [1-4].
Although several surgical options exist for the treatment of B2 glenoids, no clear guidelines that would help dictate management for the presenting patient have been established. Recent advancements have seen surgeons use anatomic total shoulder arthroplasty (aTSA), reverse shoulder arthroplasty (RSA), and, in rare cases, hemiarthroplasty to treat this pathology, incorporating bone grafting, eccentric reaming, or augmented implants [1-4]. The optimal treatment approach for B2 glenoids remains a topic of debate, particularly in light of the plethora of options available in the repertoire of shoulder surgeons and recent advances in surgical management. This review covers the current state of knowledge regarding B2 glenoids, the associated challenges, and the different management options for its treatment.
GLENOID DEFORMITY AND ETIOLOGY
In 1982, Neer was the first to describe the changes that occur in the glenohumeral joint due to osteoarthritis [5]. This was followed by Walch et al. [6] in 1999, who provided a classification model of glenoid morphology in glenohumeral osteoarthritis. The classification developed by Walch et al. used preoperative computed tomography (CT) scans to identify four main glenoid types (A, B, C, and D) based on glenoid wear patterns and the existence of humeral head subluxation [2,6].
The type B glenoid, which is marked by posterior humeral head subluxation with asymmetric wear, can be divided into three classifications: B1, B2, and B3 [6]. The B2 subgroup deformity is brought on by asymmetrical bone and cartilage degeneration, similar to that which occurs after posterior humeral head dislocation [7]. This subgroup has a variable range of severity and its posterior glenoid wear pattern produce a characteristic B2 glenoid appearance (Fig. 1) [7,8]. It contains the paleoglenoid, which represents the preserved premorbid anterior glenoid fossa and the neoglenoid, which is caused by varying degrees of posterior glenoid bone loss (Fig. 1) [7,8]. The latter occurs posteriorly in conjunction with humeral head translation, which ultimately results in a triad of B2 glenoids: glenoid biconcavity, acquired glenoid retroversion, and posterior subluxation of the humeral head [8].

The characteristic biconcave appearance of the biconcave (B2) glenoid, defined by the maintenance of a paleoglenoid and the formation of a neoglenoid due to posterior glenoid wear.
Although the neoglenoid covers a mean of 44% of the glenoid surface area, its extent in relation to the paleoglenoid varies greatly, with a depth of erosion of approximately 4 to 5 mm [3,9,10]. The asymmetric loading created by mismatches in the radius of curvatures of the neoglenoid (which has a mean of 37 mm), the paleoglenoid (a mean of 34 mm), and the humeral head (a mean of 32 mm) can lead to changes in the surface morphology of both the glenoid and the humeral head [11]. The humeral head subluxation in B2 glenoid shoulders varies depending on whether the humeral head is positioned along the scapular axis (humeroscapular) or perpendicular to the glenoid center [7]. The glenoscapular anatomy’s variable morphology, and the orientation and shape of the glenoid vault in relation to the scapular body in particular, can affect subluxation and version measurements [10]. As such, it has been shown that a direct correlation between glenoid retroversion and humeral head subluxation in reference to the scapular centerline exits [10].
DIAGNOSTICS
Many imaging modalities can diagnose B2 glenoids. Plain radiographs are commonly used by surgeons to evaluate the glenoid, yet certain issues limit the utility of this modality (Fig. 2) [12]. Conventional axillary X-rays reportedly overestimate glenoid retroversion on plain radiographs 86% of the time and offer low interobserver reliability (coefficient of correlation, 0.77) [12]. However, several authors have concluded that, compared with CT scans, high-quality plain axillary radiographs are sufficient to categorize glenoid morphology [13,14].
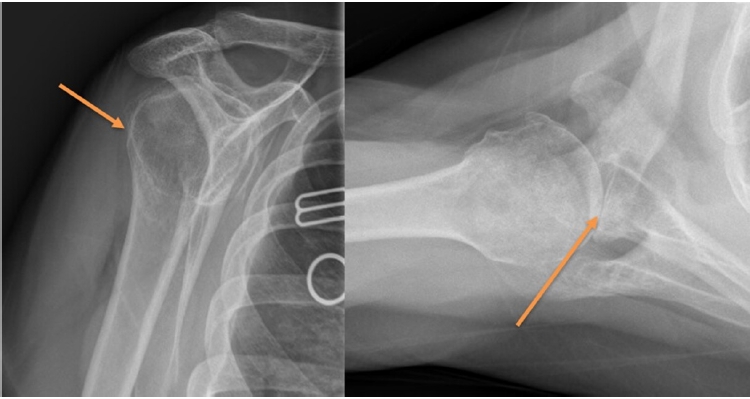
Plain radiographs have traditionally been used for initial assessment of B2 glenoids. Scapular Y view (A) can help in assessing posterior subluxation (arrow) and axillary view (B) can help evaluate diagnose B2 glenoids and approximate degree of bone loss (arrow).
Since the first description of the Walch classification, the CT scan has remained the gold standard for glenoid morphology assessment [6]. CT has achieved improved version assessment accuracy when compared with plain radiography [12]. A method described by Friedman et al. [15], in which a line between the medial border scapular tip and the center of the glenoid is referenced for the scapula axis on axial CT, is frequently used to estimate the two-dimensional (2D) glenoid version. In addition, Rouleau et al. [16] described three additional reference lines on the glenoid that are suitable for evaluating the version of the B2 glenoid: the neoglenoid, the paleoglenoid, and the intermediate glenoid, with the latter being demonstrated to be the most reliable method for measurement of the B2 glenoid version. Nevertheless, rotation of the scapula in both the sagittal and coronal planes can change the version measurements by as much as 10° [17], highlighting the potential need for three-dimensional (3D) CT scans that can correct the image orientation according to the scapular plane [18,19]. When comparing 2D and 3D CT scans, 35% of 2D images differed by 5° to 10°, and 12% had greater than 10° of difference in the version when compared with their corresponding 3D images [19]. However, overestimation of glenoid inclination and retroversion is reduced when the axis of a 2D CT slice is adjusted to the plane of the scapula [9]. Nevertheless, 2D CTs should be interpreted cautiously if they do not include 50% of the scapula [18]. As for 3D scans, the highest accuracy has been achieved by defining both premorbid and pathologic glenoid anatomy [19-23].
Magnetic resonance imaging (MRI) in shoulder assessment permits accurate evaluation of the soft tissue surrounding the joint, particularly the rotator cuff, while eliminating exposure from ionizing radiation [8]. This imaging modality can assess glenoid bone loss and version more accurately than can plain radiographs (Fig. 3) [24,25]. However, in a comparison of the accuracy of MRI and CT scans in the assessment of glenoid version and deformity, Lowe et al. [26] found no statistically significant differences in version assessment, but inferiority of MRI to CT in B2 glenoid identification. MRI may therefore be superior to plain radiographs in the assessment of glenoid morphology, but it is less reliable and accurate when compared with CT scans.
MANAGEMENT OPTIONS
Management options for B2 glenoids have varied throughout the years. Hemi-arthroplasty has fallen out of favor in recent years, following advancements in RSA and aTSA that led to improved outcomes. However, hemiarthroplasty may still play a role in the management of this pathology. Here, we present the surgical management options for B2 glenoids, while noting the use of reaming, bone grafting, and augmented components in each surgical modality.
HEMIARTHROPLASTY
Eccentric Reaming
The outcomes of hemiarthroplasty for the treatment of shoulder osteoarthritis are significantly affected by preoperative risk factors, with glenoid wear, and specifically B2 glenoid deformity, chief among them. Levine et al. [27] conducted a retrospective study of 30 patients who underwent hemiarthroplasty for osteoarthritis with an average follow-up period of 29 months. Patients were distributed into two groups based on glenoid types: type 1 and type 2 [27]. Type 1 glenoid was characterized by a concentric bony surface and degenerated cartilage but no significant bone loss or flattening [27]. Type 2 glenoids had no cartilage surface and a loss of concentric bony surface due to uneven bone loss [27]. The authors reported that patients with type 2 glenoid morphology had lower American Shoulder and Elbow Surgeons (ASES) scores and experienced less improvement in active elevation and active external rotation after surgery compared with those with type 1 morphology [27]. A follow-up study involving 27 of these patients after 17.2 years found that type I glenoid patients had superior outcomes compared with type 2 glenoid patients, with the former scoring higher in average EuroQol scores at final follow-up. In addition, revision rates were significantly higher in the type 2 group, and revised cases with type 1 glenoids had better mean Neer scores and ratings than did revised cases with type 2 glenoids [28].
Subsequent studies, including one by Iannotti and Norris [29], have also shown that patients with eccentric glenoid wear can achieve superior outcomes with total shoulder arthroplasty compared with hemiarthroplasty. Hasan et al. [30], who studied the failures of 64 hemiarthroplasties and 74 total shoulder arthroplasties, found that 42% of patients with failed hemiarthroplasties had significant glenoid erosion. Patients with either concentric or eccentric glenoid wear both had worse outcomes over time, but those with eccentric glenoids had even worse results in long-term follow-up. Likewise, a study by Smith et al. [31] on 50 shoulders found that the average age- and sex-adjusted Constant scores for patients with B2 glenoids were lower than for patients with A1 glenoids and B1 glenoids at 30-month follow-ups, with a higher rate of revision arthroplasty reported in the B2 glenoid group. These findings highlight the limitations of hemiarthroplasty in managing eccentric glenoid wear.
Ream and Run
The ream-and-run (RnR) technique has been recommended by multiple authors to achieve a concentric glenoid and reposition the humeral head while preserving the native glenoid. After an RnR, a higher level of function can be achieved, although a long recovery time and full commitment to the process are required [32]. The presence of glenoid wear, retroversion, biconcavity, and posterior de-centering of the humeral head on the glenoid do not disqualify patients from undergoing an RnR procedure, as these features are correlated with increased rates of failure of the glenoid component following aTSA, and their presence may make the RnR technique a more favorable option [32,33]. In addition, patients with B2 glenoids who undergo hemiarthroplasty remain at risk of progressive glenoid erosion due to localized posterior pressure, which can cause ongoing wear. However, if the RnR technique is used to create a single concentric concavity, joint forces become distributed over a larger area, reducing the concentrated pressure on the joint and decreasing the risk of erosion [32].
While clinical reports exploring the utility of the RnR for B2 glenoids have shown promise, results remain equivocal. Matsen et al. [34] conducted a study in 2015 that explored 28 unrevised shoulders diagnosed with the arthritic triad (glenoid biconcavity, glenoid retroversion, and posterior displacement of the humeral head) that had been treated with the RnR procedure. The RnR procedure led to improved centered position of the humeral head with respect to the glenoid and significantly higher simple shoulder test (SST) scores at a mean follow-up of three years [34]. However, several other studies encountered limitations with this technique in the surgical setting of B2 glenoid management. Lynch and colleagues found that a significant percentage of patients experienced progressive medial erosion and recurrent posterior glenoid erosion in early follow-ups to an RnR procedure [35]. In addition, Weldon and colleagues reported that resection of labral tissues and glenoid cartilage, as seen in the RnR technique, can result in decreased stability, highlighting the need for caution when performing hemiarthroplasty with or without glenoid reaming in patients with B2 glenoid morphology [36].
ANATOMIC TOTAL SHOULDER ARTHROPLASTY
Eccentric Teaming Plus Anatomic Total Shoulder Arthroplasty
In the setting of aTSA with eccentric reaming, the amount of bone loss and version correction required before reaming must be determined as many studies have proposed a limit to how much correction can be made when reaming the anterior high side. Some studies have shown that reaming can make up for up to 8 mm of bone loss in the posterior glenoid and correct version up to approximately 15° [37-42]. Another study by Sabesan et al. [10] proposed a limit for versions corrections within a range of 6° of retroversion (rather than 0°), which also falls within the remaining 10° of retroversion that is biomechanically preferred [43]. Exceeding these limits can cause a risk of excessive medialization of the joint line, reduced rotator cuff effectiveness, peg perforations, and increased micromotion [11]. Sowa et al. [44] showed that glenoids implanted with corrected reaming was associated with less micromotion compared with specimens with uncorrected reaming and augmented glenoids. It is therefore critical to carefully examine the amount of bone loss and version correction required before reaming so that, if sufficient bone stock is available, retroversion correction can be favored. However, Service et al. [45] in 2017 presented contrasting data by comparing the outcomes of glenoid components that were implanted at <15° or >15° of final retroversion. The results showed that, apart from increased peg perforation in the more retroverted group, no significant differences in clinical or radiographic outcomes were reported [45]. Orvets et al. [46], who recently conducted a clinical study involving aTSA corrective reaming for patients with B2 glenoids, found that treatment of shoulders with an average of 18° of preoperative retroversion and 67% humeral head posterior subluxation resulted in favorable clinical outcomes. All followed-up patients had an improved ASES and SST scores. Additionally, using a preoperative retroversion threshold of 20° did not affect the rate of radiographic lucency or revisions due to loosening or instability at 50-month follow-ups [46]. Consequently, patients with severe glenoid retroversion can still be candidates for aTSA with corrective reaming. However, glenoid failure may be correlated with initial glenoid component seating [46]. If so, reaming during preparation should result in a perfectly congruent surface; otherwise, biconcavity can hinder seating and insufficient bone preparation can lead to insufficient support, leading to edge loading and increased micromotion [47].
Bone Grafting Plus Anatomic Shoulder Arthroplasty
Bone grafting in aTSA is one of the most commonly suggested approaches to overcoming B2 glenoid defects, simultaneously achieving version correction, preserving bone stock, enhancing implant support, and minimizing reaming and joint-line medialization [7]. It is generally reserved for highly active young patients with increased retroversion for whom other means such as excessive reaming or RSA would be inadequate [7]. The resected humeral head is used as a source for the harvested bone graft, which is matched to the defect of the neoglenoid, prepared, and fixed using two cortical screws for compression [48]. Patients undergoing bone grafting in aTSA have generally experienced positive clinical outcomes, with complications including radiolucency, failure of graft incorporation, and graft resorption [7]. In a retrospective review of 19 patient with shoulders that underwent bone graft augmentation for deficient glenoid bone stock during aTSA, Neer and Morrison [49] reported mostly positive outcomes, with 84% excellent and 5% satisfactory outcomes, and only 11% outcomes in the limited-goals category. Radiographic analysis showed successful osseous support with no radiolucent lines in 68% of cases and incomplete radiolucent lines in 32% of cases, leading to a conclusion that bone graft augmentation was effective in allowing for glenoid implantation [49]. Steinmann and Cofield [50], who reported on humeral head bone grafting in 28 patients with segmental glenoid wear during aTSA, using various types of glenoid prostheses, observed improvement in range of motion, with 46% excellent, 36% satisfactory, and 18% unsatisfactory outcomes, and radiographic findings showing radiolucency in 54% of cases, with only two of the three radiographically loose glenoid implants being symptomatic [50]. Hill and Norris [51] conducted a long-term evaluation of bone grafting in 17 shoulders during aTSA for glenoid volume restoration and version correction. Their study included shoulders with various etiologies of glenohumeral arthritis, and the outcomes demonstrated that proper graft fixation was achieved in 14 of 17 cases [51]. However, they also observed a 29% failure rate associated with symptomatic glenoid loosening [51]. In a study of 12 patients with severe glenoid retroversion who underwent aTSA with autogenous bone grafting [52], Sabesan et al. [52], reported good or excellent range-of-motion Penn scores in 83% of patients, but noted complications related to graft healing and fixation in 17% of patients, as well as hardware complications in two patients. These results were consistent with prior studies indicating bone grafts resulted in substantial clinical and radiographic improvement, but raising concerns about graft-related complications [52].
Klika et al. [52] reported on the outcomes of bone grafting in the shoulders of 25 patients, 12 of whom had B2 glenoids, who underwent aTSA with a mean follow-up of 8.7 years. Of the 12 B2 glenoids cases, eight showed excellent clinical outcomes and two showed satisfactory outcomes, whereas two required revision surgery due to aseptic glenoid loosening [53]. Graft healing was incomplete in five of the 12 cases, but all cases showed excellent clinical outcomes [53]. A study by Nicholson et al. [48] in 2017 on 28 shoulders (15 with B1 glenoids and 13 with B2 glenoids) undergoing bone grafting and aTSA showed 100% graft incorporation without any clinically significant complications. However, Walch et al. [54] encountered a high rate of bone-grafting complications in patients undergoing aTSA for B2 glenoids. Posterior humeral head autografting was associated with significantly worse clinical outcomes, including reduced active elevation, Constant scores, mobility, strength, radiolucent lines, and complications such as graft collapse and posterior dislocation, leading the authors to caution against the use of this technique [54].
Augmented Implants and Anatomic Shoulder Arthroplasty
A novel method for dealing with B2 glenoids includes the use of a posteriorly augmented glenoid baseplate in aTSA [55]. This method comes with the advantage of correcting retroversion and avoiding joint-line medialization due to loss of bone stock, as in excessive reaming, therefore maintaining favorable biomechanical qualities while also being a better construct than those that rely on bone grafting [55]. Early designs of the augmented baseplate had disappointing outcomes and were discontinued from clinical use [55]. Commonly used designs provide correction using geometry at the bony interface, using a stepped, wedged, or hemi-wedged model [55]. A study by Favorito et al. [56] focused on the shoulders of 20 patients with osteoarthritis and posterior glenoid bone loss who were treated with aTSA using the Step-Tech (DePuy Orthopedics) glenoid augment, with a mean follow-up of 36 months. Statistically significant improvement was noted in forward flexion, external rotation, visual analog scale (VAS) score, Western Ontario Osteoarthritis of the Shoulder (WOOS) score, 36-Item Short Form Survey (SF-36) physical component summary score, perfect glenoid seating scores, and a mean Lazarus score of 0.53 [56]. Of the 20 shoulders, 12 showed osseous integration between the central-peg flanges, six had bone adjacent to the central-peg flanges but without identifiable osseous integration, and one showed osteolysis [56]. Two patients experienced a total of 3 episodes of prosthetic instability, requiring surgical intervention [56]. Another study by Stephens et al. [57] of 21 patients with B2 or C glenoid morphology who underwent aTSA with the Step-Tech glenoid augment showed similarly positive outcomes. There was significant improvement in range of motion, VAS pain scores, ASES scores, and SST scores, as well as radiographic improvement in the glenoid version, humeral scapular alignment, and humeral glenoid alignment [57]. All patients showed evidence of ingrowth around the central peg, and component seating was complete in 19 patients [57]. No complications were reported, and no clinical or radiographic failures were observed [57]. Another recent study by Iannotti et al. [58] explored 92 patients with 42 A1 glenoids, 29 B2 glenoids, and 21 B3 glenoids, all of whom were treated with the Step-Tech glenoid augment at a minimum follow-up of 2 years. The authors reported no differences in the occurrence of central-peg osteolysis between patients with B2 glenoids treated with the augmented component (10%) and those with A1 glenoids treated with the standard component. No difference was noted in the postoperative version and inclination of the glenoid component between the groups, but B3 glenoids were linked to more medialization of the component in comparison with A1 and B2 glenoids [58]. Recently, a study by Gutman et al. [59] in 2023 assessed 50 patients (41 with B2 glenoids and 9 with B3 glenoids) who underwent aTSA using Step-Tech augmented glenoids. At a mean follow-up of 42 months, a single patient had center-peg osteolysis and another had glenoid component loosening [59]. The average postoperative single assessment numeric evaluation (SANE) score was 94, while the average postoperative VAS score was 0.5 [59]. Posterior subluxation relative to the glenoid face was moderately associated with a lower SANE score [60]. In regard to wedged designs, Wright et al. [60] compared 24 patients with significant posterior glenoid wear who underwent aTSA with Equinoxe (Exactech) posterior augmented glenoid against those without posterior glenoid erosion who underwent non-augmented aTSA, with a minimum follow-up of 2 years. All patients experienced significant improvement in pain and function, and no surgical complications were reported in either group [60]. Among the patients with a posterior augmented glenoid, 60% had radiolucent lines with an average radiographic line score of 1.10, compared with the nonaugmented glenoid group, in which 33% of patients had radiolucent lines with an average radiographic line score of 0.438 [60]. In the posteriorly augmented group, two humeral heads showed superior subluxation in a Grashey view, and three were anteriorly subluxated on the axillary lateral view, with no posterior subluxations observed [60]. In a 2022 study by Garrigues et al. [61], 86 B2 glenoids treated with the PERFORM+biconvex augmented glenoid (Tornier-Stryker upper extremity) were assessed with a mean follow-up of 35±10 months. Significant improvement was noted in range of motion, glenoid retroversion, posterior subluxation and decentering, VAS score, SANE score, Constant score, and ASES score [61]. Of 86 patients, 79 had a Lazarus score of 0, indicating no radiolucency around the peg or keel, and only one patient required revision surgery [61]. These results confirm that aTSA with augmented baseplates can provide a reliable management option for many B2 glenoid patients.
REVERSE SHOULDER ARTHROPLASTY
RSA is a promising modality for the management of B2 glenoid deformities, offering inherent stability and favorable biomechanics, given its semi-constrained nature [62]. The screw fixation of the glenoid baseplate also permits easier insertion of bone grafts [63]. Several techniques, including eccentric reaming, bone grafting, and baseplate augmentation, allow the surgeon to adapt the construct to patient-specific anatomy.
Eccentric Glenoid Reaming Plus Reverse Shoulder Arthroplasty
Eccentric glenoid reaming is one of the simplest techniques for correcting small variations in the glenoid version. It requires minor modification of an essential surgical step, making it cost-efficient in the operating room [62]. In a retrospective study by McFarland et al. [64], 42 patients underwent RSA with glenoid reaming without bone-grafting. The mean follow-up period was 36 months, and the patients underwent preoperative and postoperative assessment [64]. They were evaluated objectively by a physical examination with goniometric evaluation of shoulder range of motion, radiographic assessment of baseplate loosening and scapular notching, and subjective assessment by VAS, ASES, the L’Insalata score, SST, WOOS index, the Constant-Murley, and the SF-36 scores. Postoperatively, all the patients showed improved patient-reported outcomes and range of motion (P<0.001) [64]. No studies to date have reported on the maximal limits of correction that can be achieved with RSA [62].
Glenoid Bone Grafting Plus Reverse Shoulder Arthroplasty
Glenoid bone grafting is easier in RSA than in aTSA because the former provides a stronger baseplate fixation with a central peg or a central screw with several peripheral locking screws [62]. Boileau et al. [65] performed a retrospective study in which an autologous trapezoidal bone graft harvested from the humeral head was used to compensate for glenoid deficiency and erosion. The study included 54 patients with a mean follow-up of 36 months. All patients were evaluated pre- and postoperatively by physical examination for assessment of range of motion, and by the Constant-Murley score and subjective shoulder value (SSV) assessment. There was a statistically significant (P<0.001) improvement in the Constant-Murley score, from 31 to 68, and the SSV assessment, from 30% to 80% [65]. In another retrospective study by Jones et al. [66], 44 patients requiring glenoid bone grafting during RSA were evaluated pre- and postoperatively by four shoulder-specific outcome instruments, physical examination with goniometric measurements, and radiologic evaluation. The average follow-up duration was 40.6 months. A statistically significant improvement (P<0.001) was seen in all functional outcomes postoperatively. However, no significant differences in outcomes between patients who had allografts and those who had autografts were evident [66].
Glenoid Baseplate Augmentation Plus Reverse Shoulder Arthroplasty
The use of augmented baseplates in RSA for patients with B2 glenoids has shown great potential. In a study by Jones et al. [67], 80 patients who received primary RSA for significant glenoid defects were assigned to two cohorts: 41 to a bone-grafting cohort and 39 to a glenoid baseplate–augmentation cohort. The average follow-up duration was 28.3 months for the baseplate-augmentation cohort and 34.1 months for the bone-grafting cohort [67]. Patients were assessed pre- and postoperatively using the SST scores, University of California-Los Angeles shoulder score, ASES scores, Constant scores, and Shoulder Pain and Disability Index scores, as well as measurements of range of motion [67]. All patients achieved significant improvement in functional outcome scores, pain, and range of motion. The augmented-baseplate cohort achieved a lower scapular notching rate (10%) in comparison with the bone-grafting cohort (18.5%) [67].
CONCLUSIONS
B2 glenoids are a challenging pathology for shoulder surgeons worldwide, as significant glenoid defects can increase the risk of complications in shoulder osteoarthritis patients, and lead to high failure rates. Diagnosing B2 glenoids often relies on imaging and radiographic evaluation, with CT often considered the gold standard for assessing degree of retroversion and posterior subluxation. Management of B2 glenoids has long been the subject of debate, as many surgical options have emerged to treat this challenging pathology. These include hemiarthroplasty, aTSA and RSA, with incorporation of corrective reaming, bone grafting, and augmented components gaining increasing traction over the recent years. Surgeons should conduct comprehensive clinical and demographic evaluations of the presenting patient and appropriately assess imaging findings before choosing a procedure. It is also important for surgeons to educate their patients on all the available options and to involve them in the decision-making process, in order to ensure appropriate expectations and high levels of patient satisfaction.
Notes
Author contributions
Conceptualization: MYF, MD, JM, ERH, JPR, PB, JAA. Data curation: MYF. Investigation: MYF, JM, ERH, JPR, PB. Methodology: MYF, MD, JM. Supervision: JAA. Writing – original draft: MYF, MD, JM, ERH, JPR, PB, JAA. Writing – review & editing: JAA.
Conflict of interest
JAA would like to disclose royalties from: DJO Global, Zimmer-Biomet, Smith and Nephew, Stryker, Globus Medical, Inc.; research support as a PI from: Lima Corporation - Italy, Orthofix, Arthrex, OREF; royalties, financial or material support from: Wolters Kluwer; and board member/committee appointments for: American Shoulder and Elbow Society, Pacira.
Funding
None.
Data availability
None.
Acknowledgments
None.

