 |
 |
- Search
| Clin Shoulder Elb > Volume 26(2); 2023 > Article |
|
Abstract
Background
Preoperative traditional software planning (TSP) is a method used to assist surgeons with implant selection and glenoid guide-pin insertion in shoulder arthroplasty. Mixed reality (MR) is a new technology that uses digital holograms of the preoperative plan and guide-pin trajectory projected into the operative field. The purpose of this study was to compare TSP to MR in a simulated surgical environment involving insertion of guide-pins into models of severely deformed glenoids.
Methods
Eight surgeons inserted guide-pins into eight randomized three-dimensional-printed severely eroded glenoid models in a simulated surgical environment using either TSP or MR. In total, 128 glenoid models were used and statistically compared. The outcomes compared between techniques included procedural time, difference in guide-pin start point, difference in version and inclination, and surgeon confidence via a confidence rating scale.
Results
When comparing traditional preoperative software planning to MR visualization as techniques to assist surgeons in glenoid guide pin insertion, there were no statistically significant differences in terms of mean procedure time (P=0.634), glenoid start-point (TSP=2.2±0.2 mm, MR=2.1±0.1 mm; P=0.760), guide-pin orientation (P=0.586), or confidence rating score (P=0.850).
Conclusions
The results demonstrate that there were no significant differences between traditional preoperative software planning and MR visualization for guide-pin placement into models of eroded glenoids. A perceived benefit of MR is the real-time intraoperative visibility of the surgical plan and the patient’s anatomy; however, this did not translate into decreased procedural time or improved guide-pin position.
Computer-assisted solutions for preoperative planning in orthopedic surgery have increasingly shown measurable benefits to both surgeons and patients [1-3]. Current research is focused on advancing these concepts from the preoperative to a perioperative, real time use for improved surgical execution. One way this can be accomplished is via Mixed reality (MR), a concept in which virtual objects or holograms are digitally superimposed into the real world [4]. These holograms are anchored in reality allowing the user to interact and manipulate them with voice recognition technology or via hand gestures; for example, a hologram can be rotated, enlarged, zoomed in on, or moved to a different location in the real world (Fig. 1).
Presently, there is little evidence exploring the use of MR in shoulder arthroplasty. One case report describes the first use of such a system in a surgical environment [5], and a recent feasibility study explores the use of a head-mounted display in the surgical planning of a reverse shoulder arthroplasty glenoid baseplate [6].
Traditional three-dimensional (3D) preoperative computer planning has been gaining traction as an effective presurgical visualization and conceptualization method to aid surgeons during shoulder arthroplasty. This method of preoperative planning utilizes 3D anatomical reconstructions of the patient’s native shoulder anatomy via a computer software system, reliably providing information about glenoid version/inclination, the glenoid wear pattern, and humeral head subluxation. This information allows the surgeon to appropriately plan glenoid component positioning and to subsequently translate this preoperative plan into the operating room. Numerous implant manufacturers now offer surgical planning software. However, to successfully integrate the preoperative plan into the surgical theatre in real time requires the use of patient-specific instrumentation, which can be time-consuming and costly to manufacture, or optical navigation. An optical navigation system requires additional steps of intraoperative calibration and registration, which can increase surgical time as well as cost due to the specialized tracking equipment required [7]. An alternative to overcoming these challenges is the use of intraoperative MR visualization. A portable head-mounted display (headset) provides a 3D holographic view of a patient’s preoperative plan in the surgical field and allows for real-time user interaction and manipulation while remaining sterile (Fig. 1). The headset is optically transparent with a self-contained holographic computer, which overlays the holographic image of the preoperative plan without blocking the real environment (Fig. 2).
It is logical to postulate that successful integration of this actively evolving technology has the potential to improve surgical accuracy during shoulder replacement surgery, decrease operative time, and increase surgeon confidence, all of which may contribute to improved patient outcomes and prolonged implant longevity. As such, the purpose of this study was to compare traditional 3D preoperative computer software planning to MR visualization in an idealized simulated surgical environment using 3D-printed glenoid models, where surgeon subjects inserted guide-pins into models of severely deformed glenoids. The two techniques, traditional software planning (TSP) and MR, were compared in their ability to assist surgeons with idealized guide-pin insertion, assessing guide-pin entry point on the glenoid, and angular orientation. Our hypothesis was that MR visualization would have a lower overall procedural time, improved guide-pin positioning and result in greater user confidence than TSP.
This study was approved by Institutional Review Board of Western University Health Science Research Ethics Board (No. R-21-074). Written informed consent was obtained from each participant after the nature of the study was explained.
After obtaining institutional ethics review board approval, a total of eight patient computed tomography (CT) scans of four left and four right shoulders that met the following glenoid erosion criteria based on Lévigne et al. [8] and Walch et al. [9] classifications were selected: two E2s, one E3, two B2s, one B3, one C and one D glenoid case. Using this patient CT data, a total of sixteen duplicate 3D glenoid specimen models for each patient (Fig. 3A) were created using additive manufacturing (Prusa i3Mk3S, Prusa Research) for a total of 128 glenoid models. The thermoplastic material used was polylactic acid, with a setting of two shells, 25% infill and a 3D gyroid infill pattern to represent differences in density between cortical and cancellous bone in a natural glenoid as closely as possible.
The glenoid models were attached to a baseplate including coracoid and acromion anatomical features (left and right available for left and right glenoids), which was mounted to a work-bench fixture for stability (Fig. 3A). Once the models were secured to the fixture, they were encapsulated in a foam housing (Sawbones, Pacific Research Laboratories Inc.) mimicking the soft tissues of the shoulder (Fig. 3B) with an opening anteriorly for the deltopectoral approach. During testing, an assistant provided retraction to emulate a glenoid exposure during arthroplasty as closely as possible (Fig. 3C).
Eight surgeon testers of different experience levels, including two senior surgeons, two surgical fellows, two senior residents and two junior residents, volunteered to participate in guide-pin insertion into the deformed glenoid models. Due to time availability and the four different levels of training (from junior to senior), eight was the minimum feasible number of surgeon testers that could be chosen for this study. For the junior residents, prior experience with shoulder arthroplasty procedures was not a requirement to participate. Each participant of matched surgical experience was randomly assigned (obtained by the RAND function in Microsoft Excel) to first use either the TSP or the MR preoperative planning strategy during simulated surgery. A 2-week interval between each testing method was provided to allow the testers to forget the experience before applying the second method.
Prior to the study beginning, the eight patient cases were planned such that an idealized guide pin entry point and trajectory was pre-determined for each unique model by an experienced, fellowship trained senior surgeon (GSA) using a commercially available presurgical software planning program (BluePrint, Wright Medical). This software program utilized the DICOM data obtained from the patient’s CT scan to create a 3D model. Total shoulder arthroplasty (TSA; n=2) or a reverse total shoulder arthroplasty (RTSA; n=6) glenoid implant was templated based on the surgical indication of the case. If a total shoulder arthroplasty was indicated, a TSA glenoid component was implanted in 0°–10° retroversion, 0°–10° inclination superiorly with >90% backside seating. The glenoid component utilized was a four-peg design with a larger central peg with fins, and three peripheral pegs. If an RTSA was indicated, a baseplate was positioned at 0° version, neutral inclination and at >80% backside seating. These eight planned cases represented the idealized control and were the preoperative plans that the surgeon testers were asked to replicate in the simulated surgical environment.
This study compared preoperative traditional 3D software planning (TSP) to MR visualization. The MR method was conducted through the use of an MR headset developed and manufactured by Microsoft, the HoloLens 2 (Microsoft Corp.). To allow for equal opportunity and skill-level regarding the use of MR, no tester had any prior experience in the use of HoloLens visualization prior to testing. Each tester underwent a 15-minute HoloLens training module of the HoloLens software on the use of the device to become familiar with manual manipulation of the hologram in space, such as its rotation and zoom-in and zoom-out features using simple manual gestures. Though the HoloLens permits verbal cues for manipulation of the hologram, this study assessed manual gestures only.
The surgeon testers were asked to recreate the guide-pin location and trajectory onto the glenoid models in the simulated surgical scenario using the TSP and MR techniques for assistance. The TSP method involved viewing the preoperative plan on a computer screen utilizing the preoperative planning software and set-up approximately 2 meters away from the surgical field. Once the surgeon tester deemed themselves comfortable with the plan, they moved over to the simulated surgical field for true guide-pin insertion. During guide-pin insertion, the surgeon tester could view the preoperative plan on the computer screen, however, manipulation of the TSP with hand gestures was not possible. The MR method utilized the HoloLens to project a digital hologram of the preoperative plan adjacent to the simulated operating field set-up (Fig. 2). In both circumstances, the surgeon testers had unrestricted access to the plan and the 3D images to manipulate and alter the vantage point to assist with interpretation and landmarking for eventual guide pin insertion.
The surgeon testers were randomly assigned to either method (TSP or MR) and were allowed unrestricted access to view the 3D scapular anatomy of each case from all vantage points to assess and landmark the trajectory of the guide-pin. The testers were also permitted to manipulate the plan; however, they were informed that in addition to accuracy, time was a recorded outcome measure. Timing started at the first reveal of the computer screen in the TSP method and after flipping down the visor of the headset in the MR method. Timing ended with a vocal cue by the tester after final insertion of the guide-pin into the glenoid model had taken place. The eight testers inserted guide-pins into eight unique glenoid models for the TSP method and inserted eight guide-pins into eight duplicate models using the MR method, for a total of 16 glenoid models per tester. This resulted in a total of 128 glenoid models containing a guide-pin for comparison.
After completing guide-pin insertion into the specimens using either method, the tester was provided with a confidence rating scale in the form of a short questionnaire and was asked to rate their confidence in guide-pin positioning and insertion pertaining to the method used. Due to a lack of literature on which to base our questions, expert opinion of three experienced shoulder surgeons was sought through an approach that has been used previously in related studies [10-12], and a set of four statements was created, which included the following: (1) The planning method helped me find the guide-pin insertion position with ease. (2) The planning method made me feel confident in my guide-pin insertion. (3) This planning method was easy to use. (4) I would use this planning method again. Participants were asked to rate their agreement with the above items on a scale of 1 (strongly disagree) to 5 (strongly agree).
Overall outcome measures of the study included (1) total individual procedural time to insert the guide-pin into the glenoid model, (2) difference in guide-pin entry point location from control, and (3) difference in orientation from the control reported as version and inclination, and (4) subjective surgeon confidence via the above confidence rating scale.
To analyze the deviation of the executed guide-pin entry point and trajectory from the planned control, the guide pin trajectory was digitized. Each specimen was rigidly fixed to an optical tracker and digitization points were collected and recorded using a tracker mounted stylus (Optotrak Certus Position Sensor Full, Northern Digital Inc.) in combination with North Digital Inc. First Principles software (Northern Digital Inc., ver. 1.2.4). Digitization of the inserted guide-pin was achieved by probing the start point, the opposite end of the guide-pin, the mounting points, and taking a trace of the guide-pin.
Analysis of the orientation and insertion of the guide-pin was achieved by importing the 3D models into SolidWorks 2020 software (Dassault Systèmes SolidWorks Corp., ver. 28.3.0.0086). Measurements were taken to determine the location of landmarks to establish a glenoid coordinate system which was partially based on previous protocols [13].
Data from both the control and the digitization of the guide-pin insertions were imported to MATLAB software (The MathWorks Inc., ver. 9.7.0.1190202) for comparison. Analyses were conducted to compare the orientation of the guide-pin with respect to the glenoid coordinate system (X=anterior/posterior, Y=inferior/superior, Z=medial/lateral) as well as determining the entry point of the pin.
Statistics were calculated using IBM SPSS ver. 27.0 (IBM Corp.) and a multivariate analysis of variance (MANOVA) was employed to compare the sample means for version, inclination and point location for each specimen, tester and method, and order of method. The same statistical test (MANOVA) was applied to compare the sample means for procedural time in the same categories. A P-value less than 0.05 was considered to be statistically significant. Descriptive statistics are provided as mean±standard deviation (95% confidence interval [CI]).
All 128 glenoid models underwent testing without any material issues. The average procedural time from visualizing the presurgical plan to completion of guide-pin insertion for all specimens was 63±3 seconds (95% CI, 56–70 seconds). The mean procedure time for the TSP method was 62±4 seconds (95% CI, 53–72 seconds) and for the MR method was 64±3 seconds (95% CI, 58–70 seconds) and these were not statistically different (P=0.634). Regarding surgeon experience level, the mean procedure time for junior residents was not significantly different (P=0.120) from senior residents, at 50±3 seconds (95% CI, 44–56 seconds) and 56±2seconds (95% CI, 49–60 seconds), respectively, and regardless of method. Both times were significantly longer (P<0.020) than the time it took shoulder fellows to insert the guide-pin (39±3 seconds; 95% CI, 33–46 seconds). Among all four experience levels, senior surgeons took the longest (P<0.001) to complete the guide-pin insertion for both procedures, with an average of 109±9 seconds (95% CI, 88–131 seconds).
Procedural time also differed significantly (P=0.001) from first to second trial, regardless of what method (TSP or MR) was used first or second, with the first trial taking a mean of 69±3 seconds (95% CI, 61–76 seconds) and the second trial taking less time at 58±3seconds (95% CI, 50–66 seconds). Surgeon testers who started with the TSP method took an average of 87±6 seconds (95% CI, 73–101 seconds) and subsequently took 77±5 seconds (95% CI, 66–89 seconds) to complete the same procedure using the MR method. Surgeons who started with the MR method took an average of 50±2 seconds (95% CI, 46–54 seconds) to complete the guide-pin insertion and subsequently required 38±2 seconds (95% CI, 33–44 seconds) to complete the procedure with the TSP method. The procedural mean times based on method and surgeon experience level are listed in Table 1.
The overall mean deviation from the planned guide-pin entry point in all specimens and both methods (Fig. 4) was 2.1±0.2 mm (95% CI, 1.8–2.5 mm). Specifically, on an X-Y axis with the center going through the planned control guide-pin entry point, and with X describing the anteroposterior translation and Y describing the superoinferior translation, the average absolute distance away from the planned entry point was 2.1±0.2 mm (95% CI, 1.6–2.7 mm) in the anteroposterior and 2.1±0.1 mm (95% CI, 1.8–2.5 mm) in the superoinferior directions (P=0.972). There was no significant difference (P=0.760) in glenoid start point comparing TSP to MR at 2.2±0.2 mm (95% CI, 1.6–2.7 mm) and 2.1±0.13 mm (95% CI, 1.8–2.4 mm), respectively. Additionally, surgeon experience had no significant effect (P=0.270) on glenoid start point: junior residents (2.0±0.2 mm), senior residents (2.2±0.2 mm), fellows (2.1±0.2 mm), senior surgeons (2.1±0.3 mm). Similarly, the order of testing showed no statistically significant difference to glenoid start point location (P=0.160). The mean guide-pin glenoid start point deviations from the control in millimeters are listed in Table 1.
The mean deviation angle in version for all specimens measured was 8°±1° (95% CI, 6.3°–10.3°) and in inclination it was 8°±1° (95% CI, 6.1°–9.6°) (P=0.722). There was no statistical difference (P=0.105) between the TSP and MR methods for either version or inclination, with 9°±1° (95% CI, 7.1°–10.2°) and 8°±1° (95% CI, 6.4°–8.5°), respectively (P=0.586). Additionally, surgical experience level showed no statistically significant difference (P=0.085) in executed trajectory deviation as compared to the control: junior residents, 9°±1° (95% CI, 7.9°–10.0°); senior residents, 7°±1° (95% CI, 5.4°–9.1°); fellows, 8°±1° (95% CI, 6.3°–9.3°); and senior surgeons, 8°±1° (95% CI, 6.1°–10.6°). Furthermore, the order of testing had no significant effect on guide-pin trajectory deviation (P=0.689).
The overall confidence rating score for the TSP method was 4.0 out of a maximum score of 5.0 and it was not significantly different (P=0.850) from the MR score of 4.2. The overall confidence questionnaire ratings based on surgical method and surgical experience levels are listed in Table 1.
There is increasing interest in the integration of MR into the orthopedic operating room. Thus far, the majority of literature pertains to the use of augmented reality for educational purposes, which allows for the study of technical performance as well as telemonitoring. However, interest in incorporating this new immersive form of visualization live into the operating room to achieve accuracy in a surgical procedure is novel and growing. In recent years, such technological uptake has become more feasible due to improvements in both cost-effectiveness of MR and virtual reality devices [4].
In shoulder arthroplasty, MR visualization was introduced in the context of complicated cases in the hopes it would provide immediate value in implant positioning and save valuable surgical time [4]. This was hypothesized, in part, due to the preoperative plan being intraoperatively available in a 3D hologram form, which could be visualized and manipulated by the operating surgeon in real-time during surgery. Additionally, by having the 3D hologram of the guide-pin trajectory adjacent to the native glenoid, we theorized this readily visible resource would assist surgeons in improved guide-pin placement.
Based on the results of this study, however, our hypotheses pertaining to the benefits of MR visualization were rejected. The overall results did not reveal any statistically significant differences between the use of TSP or MR visualization for guide-pin placement, procedural time or user confidence. The lack of significant difference between results was unexpected and surprising. A potential explanation may be that all of the surgeons were first time users of the MR HoloLens headset, and as such, there may be a learning curve to realizing its benefit. For first time users, there is some level of adjustment required to acclimate to the holograms and the hand gestures required for manipulation and setup. Additionally, the simulated surgical setting did not completely simulate the challenges of glenoid exposure, and the stressors of real live surgery.
Interestingly, we found some statistically significant differences in terms of the time it took to complete the procedures. Fellows took significantly less time overall for both methods (P=0.020), while testers who were randomized to begin with the MR method appeared to have taken less time for the procedure than those who started with the TSP method; 50±2 seconds (95% CI, 46–54 seconds) for MR compared to 87±6 seconds (95% CI, 73–101 seconds) for TSP. But given that there was no statistically significant difference between the overall procedure times for TSP (62±4 seconds; 95% CI, 53–72 seconds) and MR methods (64±3 seconds; 95% CI, 58–70 seconds) (P=0.634), it appears that there are several underlying factors to consider in combination, as will be discussed in the following paragraphs.
It is important to consider the constraints of both methods. A disadvantage of the TSP method is its limited portability into the sterile operating field. The TSP method can, however, be projected onto a wall or ceiling mounted computer monitor. In using the TSP method intraoperatively, the surgeon must physically look away from the surgical site and turn the head or body towards where the monitor is located. For the HoloLens MR system, advantages include the alignment of the holographic presurgical plan to the surgical site within the surgeon’s field of view and the immediate ability to compare and correct position and alignment of guide-pin placement to the preoperative plan with a simple visual comparison without the need to turn ones’ head or body. It was therefore expected that the MR method would outperform the TSP in both procedural time and accuracy in guide-pin placement. However, the use of this novel method inevitably comes with its own set of drawbacks, which likely had an effect on the outcome measures. There were several difficulties in operating the HoloLens, which either affected tester’s ability to complete the task as required or else resulted in dissatisfaction with the system. There is a learning curve to the appropriate hand gestures needed to manipulate the holograms. The headset only responds to precise hand gestures and, as such, subtle imperfections in hand positioning or motion result in a non-response, which is time consuming and potentially frustrating for users. This is also likely the explanation for the increased time it took senior surgeons to complete the guide-pin insertion for both procedures compared to the other surgeon experience levels. While they possess more surgical experience, the increase in length of time is likely best explained by the slower adaptation to new technology.
An interesting finding of the present study was that the use of preoperative planning software resulted in junior and senior residents being able to free-hand insert a guide-pin into a deformed glenoid with a mean start point deviation of only 2.2 mm and a mean trajectory deviation of only 8°. These values were not substantially different than the consultant surgeons (2.1 mm and 8°). Overall, it appears as though some form of preoperative software planning may be beneficial to improve surgical execution. These results are in line with research by Iannotti et al. [14], which concluded that advanced patient specific instrumentation did not result in any consistent improvements over 3D preoperative planning alone. Although the final values for guide pin insertion were quite good, it must be stated that these insertions occurred in a simulated surgical set-up, without the challenges of glenoid exposure, blood in the operative field, and the inherent stress of the surgical theater.
Limitations of this study overall include that the testing was conducted in a controlled environment simulating the single step of accurate guide-pin insertion, which is one step among many in shoulder arthroplasty surgery. The challenges of soft tissue exposure of the glenoid, a known issue, were not completely assessed. It is theorized that less experienced surgeons would have greater challenge exposing the glenoid for accurate guide-pin insertion. Secondly, we developed our confidence rating scale items using expert opinion due to the paucity of available data and a lack of similar tools in the literature. To our knowledge, there are no published articles regarding the characterization of ‘confidence’ in using one presurgical planning method over another. Therefore, our question items may not represent a complete catalogue of confidence measures. Additionally, it is important to note that there were some 3D printing variations. The quality or resolution of patient CT scans as well as natural 3D printing parameters caused slight imperfections between the computer software created 3D images of the scapular specimens compared to the 3D-printed models. Finally, most implant manufacturers are providing preoperative surgical planning software at no additional cost. The HoloLens 2, however, costs from $3,500 to $5,000 at the time of publication of this article.
Overall, we still see potential benefit in integrating MR into the operating room. Further development of MR devices for surgical purposes, including navigation, needs to be investigated. Additionally, as MR applications and utilization increase, users will become familiar with the platform and will plateau in their learning curves.
The results of this study did not reveal any statistically significant differences between TSP and MR visualization for guide-pin placement, procedural time or user confidence. Nevertheless, we see benefits that can be derived from using MR in the operating room, especially for ease of preoperative plan visualization and manipulation. This benefit, however, does not translate to decreased procedural time or improved guide-pin position in our experimental model.
NOTES
Author contributions
Conceptualization: SA, NJVO, DGL, JAJ, GSA.Data curation: SA, NJVO, DGL. Formal Analysis: SA, NJVO, DGL. Funding acquisition: GSA. Investigation: SA. Methodology: SA, NJVO, JAJ, GSA. Project administration: SA, NJVO. Software: DGL, JAJ. Supervision: JAJ, GSA. Visualization: SA. Writing – original draft: SA. Writing – review & editing: SA, NJVO, DGL, JAJ, GSA.
Conflict of interest
The author (GSA) is a consultant for Wright Medical-Tornier Inc. Wright Medical donated the HoloLens 2 used in this study. No company had any input to the study design, protocol, testing, data analysis or manuscript preparation. No other potential conflicts of interest relevant to this article were reported.
Fig. 1.
A view of two surgeons conducting a shoulder replacement from the vantage point of an assistant wearing the mixed reality headset. As the surgeons operate, two digital holograms are visible. The left hologram is of the scapula with an anatomic glenoid component positioned and the right is an axial view of the patient’s computer-tomography scan.
ANAT: anatomic, GLN: glenoid, HUM: humerus, JNT: joint.
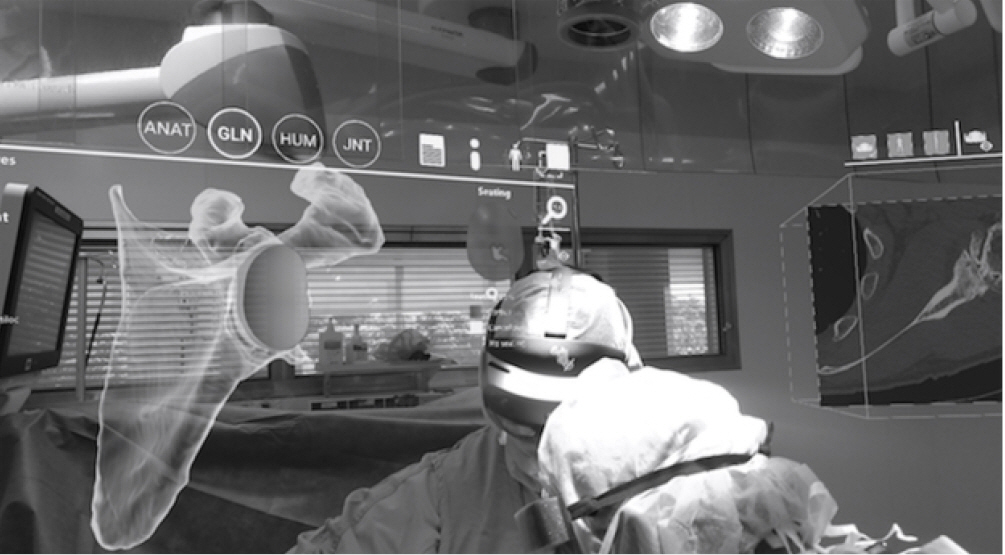
Fig. 2.
Three-dimensional (3D) holographic view of the simulated surgical field from the surgeon’s point of view using a mixed reality (MR) headset. The scapular model, the entry point, and the trajectory of the guide-pin (seen as a straight line on the glenoid face protruding outwards) are shown. The surgeon tester is holding a guide-pin loaded drill onto the 3D-printed glenoid model attempting to recreate the planned guide-pin entry point and trajectory as seen in the holographic plan above the experimental set-up.
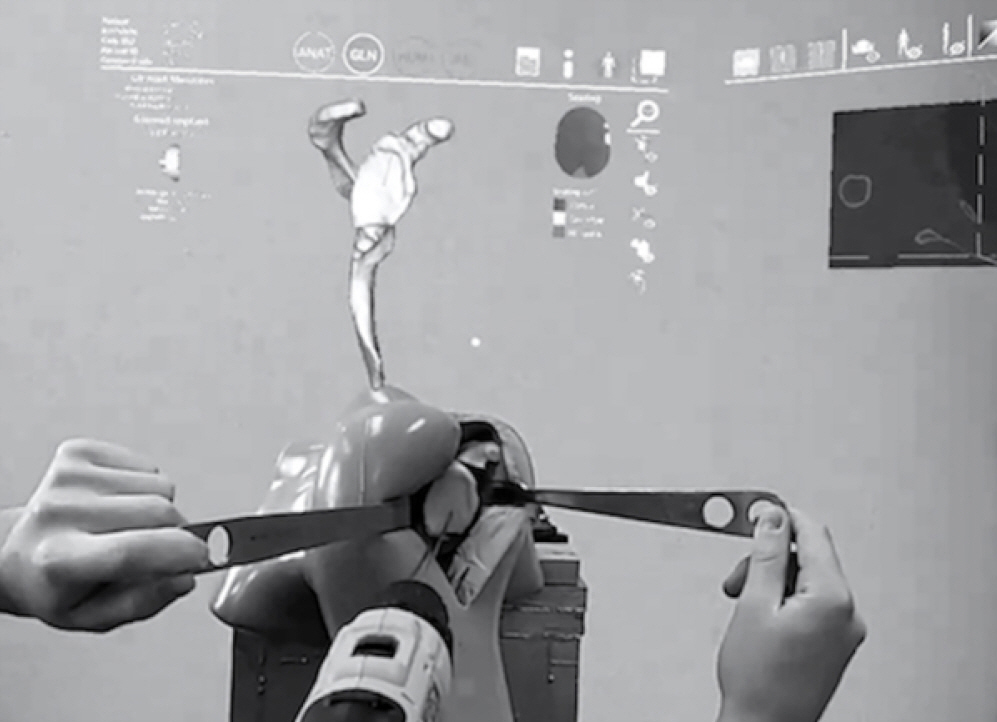
Fig. 3.
The experimental set-up. The set-up involved a three-dimensional-printed glenoid model (A) covered by a soft tissue replica of the shoulder (B) to simulate the approach (C).
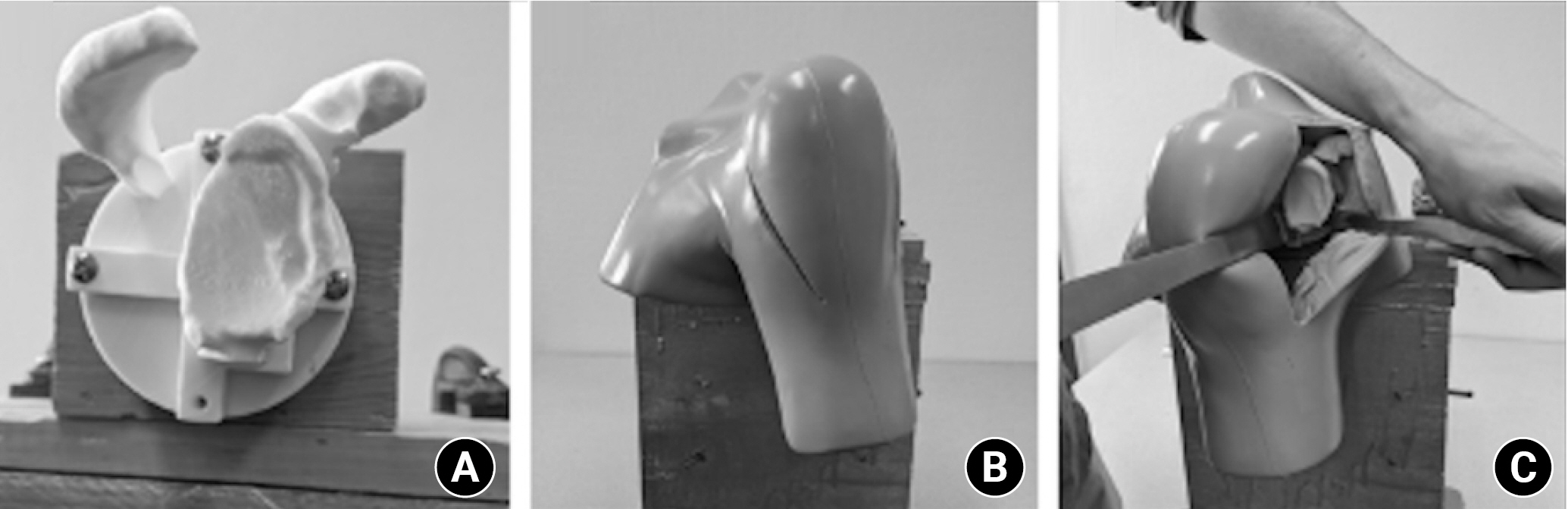
Fig. 4.
Guide-pin entry point accuracy from control, based on surgeon experience level. R1 (a) and R1 (b): first year residents, R4 (a) and R4 (b): fourth year residents, F (a) and F (b): fellows, S (a) and S (b): senior surgeons. S (positive Y-axis)=superior, A (positive X-axis)=anterior, I (negative Y-axis)=inferior, P (negative X-axis)=posterior on the glenoid face, TSP: traditional software planning, MR: mixed reality. The coordinate system center represents the control guide-pin entry point.
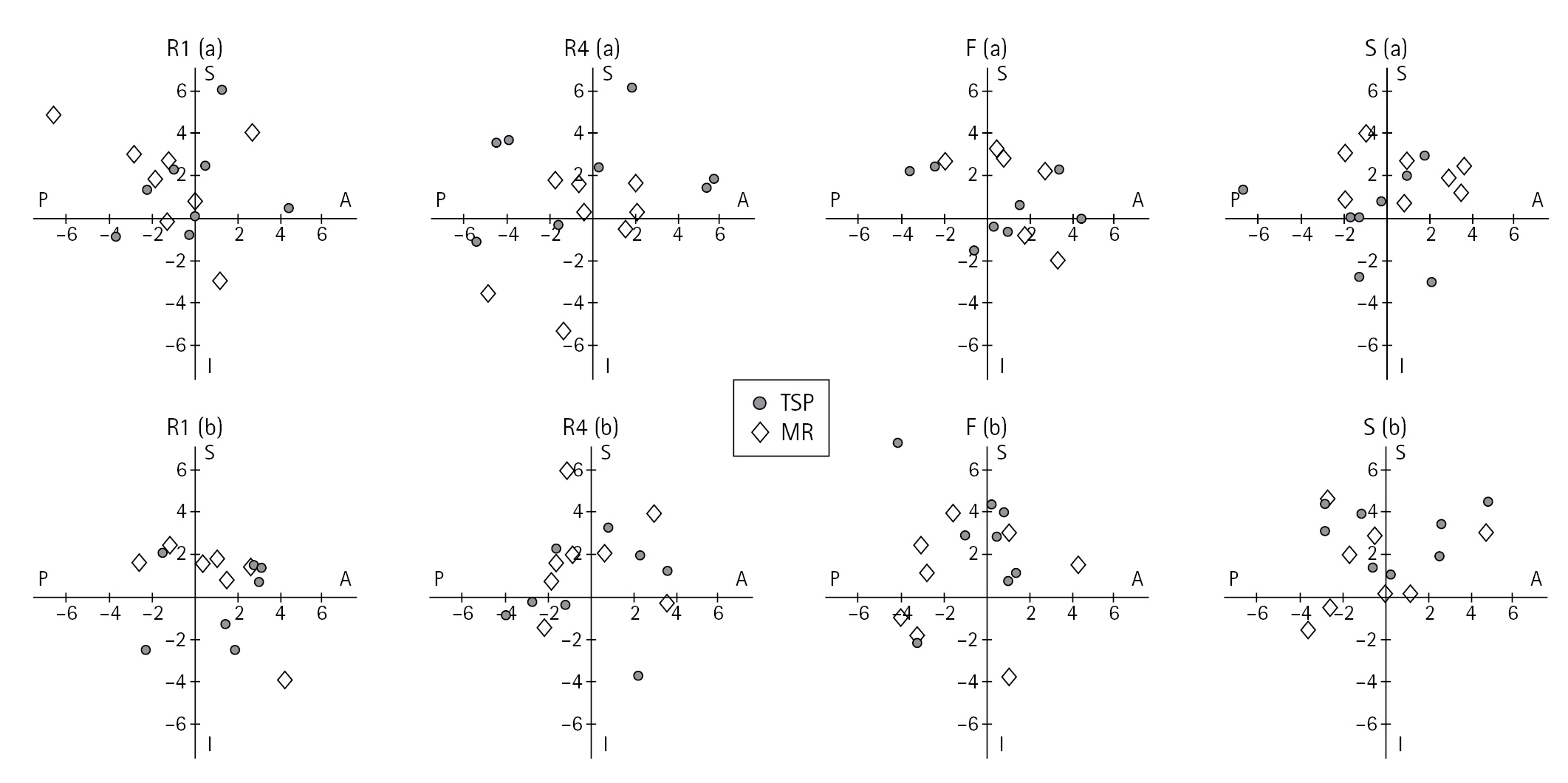
Fig. 5.
Comparison between traditional and mixed reality (MR) planning in terms of inclination (A) and version (B) error based on tester experience level. R1 (a) and R1 (b): first year residents, R4 (a) and R4 (b): fourth year residents, F (a) and F (b): fellows, S (a) and S (b): senior surgeons, TSP: traditional software planning.
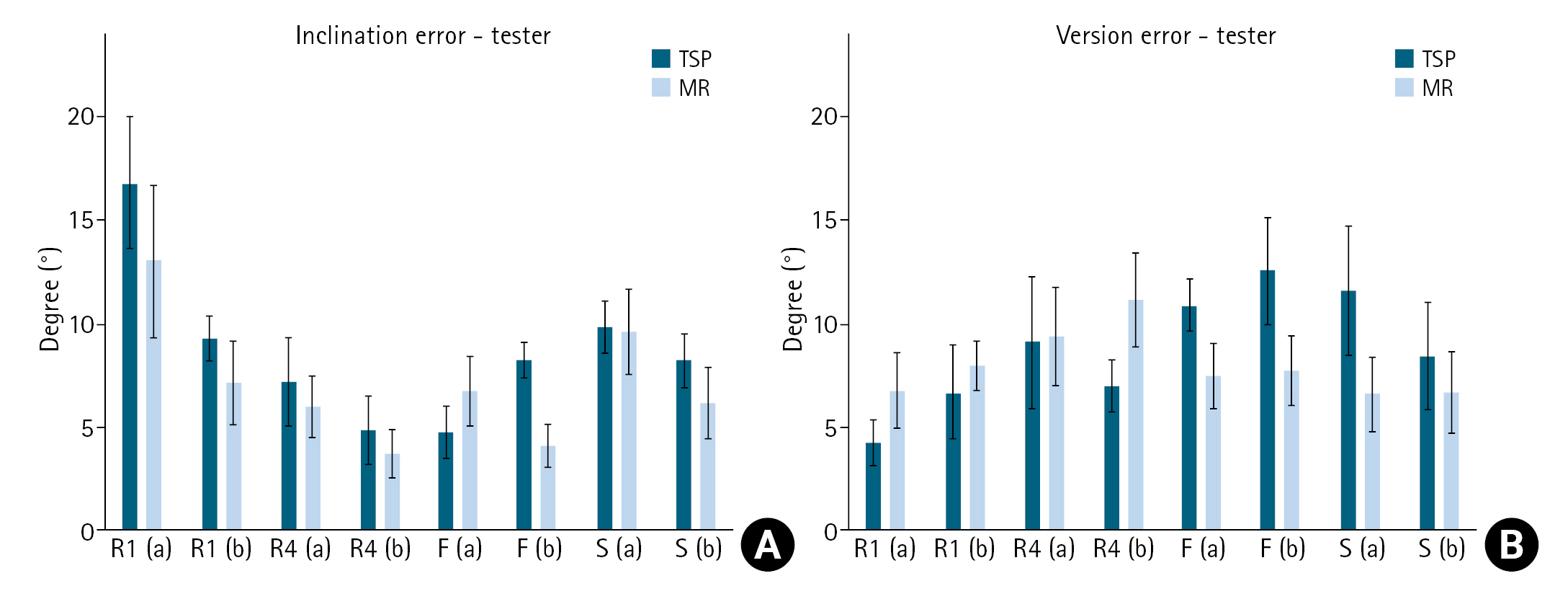
Table 1.
Testing parameters based on presurgical method and surgical experience
REFERENCES
1. Beckmann J, Stengel D, Tingart M, Götz J, Grifka J, Lüring C. Navigated cup implantation in hip arthroplasty. Acta Orthop 2009;80:538–44.



2. Hernandez D, Garimella R, Eltorai AE, Daniels AH. Computer-assisted orthopaedic surgery. Orthop Surg 2017;9:152–8.



3. Saragaglia D, Rubens-Duval B, Gaillot J, Lateur G, Pailhé R. Total knee arthroplasties from the origin to navigation: history, rationale, indications. Int Orthop 2019;43:597–604.


5. Gregory TM, Gregory J, Sledge J, Allard R, Mir O. Surgery guided by mixed reality: presentation of a proof of concept. Acta Orthop 2018;89:480–3.



6. Kriechling P, Roner S, Liebmann F, Casari F, Fürnstahl P, Wieser K. Augmented reality for base plate component placement in reverse total shoulder arthroplasty: a feasibility study. Arch Orthop Trauma Surg 2021;141:1447–53.



7. Rodríguez JA, Entezari V, Iannotti JP, Ricchetti ET. Pre-operative planning for reverse shoulder replacement: the surgical benefits and their clinical translation. Ann Joint 2019;4:4.

8. Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2008;17:925–35.


9. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty 1999;14:756–60.


10. Dandavino M, Young M, Gosselin R, Snell L, Bhanji F. Development and validation of a self-efficacy scale for clinical decision-making in general paediatrics. Paediatr Child Health 2013;18:184–8.



11. Katz S, Feigenbaum A, Pasternak S, Vinker S. An interactive course to enhance self-efficacy of family practitioners to treat obesity. BMC Med Educ 2005;5:4.



12. Mason S, Ellershaw J. Assessing undergraduate palliative care education: validity and reliability of two scales examining perceived efficacy and outcome expectancies in palliative care. Med Educ 2004;38:1103–10.










