 |
 |
- Search
| Clin Shoulder Elb > Volume 26(1); 2023 > Article |
|
Abstract
Background
Tendon degeneration contributes to rotator cuff tears; however, its role in postoperative structural integrity is poorly understood. The purpose of this study was to investigate the factors associated with postoperative structural integrity after rotator cuff repair, particularly focusing on the histology of tendons harvested intraoperatively.
Methods
A total of 56 patients who underwent primary arthroscopic rotator cuff repair between 2009 and 2011 were analyzed. A 3-mm-diameter sample of supraspinatus tendons was harvested en bloc from each patient after minimal debridement of the torn ends. Tendon degeneration was assessed using seven histological parameters on a semi-quantitative grading scale, and the total degeneration score was calculated. One-year postoperative magnetic resonance imaging was used to classify the patients based on retear.
Results
The total degeneration scores in the healed and retear groups were 13.93±2.03 and 14.08±2.23 (P=0.960), respectively. Arthroscopically measured anteroposterior (AP) tear sizes in the healed and retear groups were 24.30±12.35 mm and 36.42±25.23 mm (P=0.026), respectively. Preoperative visual analog scale pain scores at rest in the healed and retear groups were 3.54±2.37 and 5.16±2.16 (P=0.046), respectively. Retraction sizes in the healed and retear groups were 16.02±7.587 mm and 22.33±13.364 mm (P=0.037), respectively. The odds of retear rose by 4.2% for every 1-mm increase in AP tear size (P=0.032).
The prevalence of degenerative rotator cuff tears increases with age [1,2]. A cadaveric study demonstrated that the prevalence of full-thickness rotator cuff tears in subjects over the age of 60 years was 30%, compared to 6% in those under the age of 60 years [3]. As the world population is an aging population, degenerative rotator cuff tears have become an increasingly prevalent clinical problem worldwide. Surgical procedures for rotator cuff tears have evolved rapidly from open to mini-open to arthroscopic techniques [4]. Currently, arthroscopic surgery is considered the best means for treating rotator cuff tears because it provides functional results similar to those of open and mini-open surgery with fewer postoperative complications than other techniques [5].
The failure rate of postoperative structural integrity remains relatively high despite the evolution of repair techniques [6]. Failure of structural integrity after repair is observed in 20% to 94% of patients regardless of the repair technique [7]. Several studies have shown improved outcomes on postoperative imaging for patients who have healed rotator cuff tendons [8,9]. Patients with healed tendons showed increases in function and range of motion (ROM) compared to patients with failed structural integrity [10]. Patient-related factors are likely to exert a strong influence on the healing of rotator cuff tendons following surgery. In terms of postoperative structural integrity, factors to consider include the patient's age, the size and quality of the tear, tendon retraction, muscle atrophy, and fatty infiltration [8,11]. Several studies indirectly assessed tissue quality via the magnetic resonance imaging (MRI) of fatty infiltration and through the arthroscopic assessment of tissue thickness and mobility, and observed significant differences between healed and retear groups [8,11]. However, histological assessments of the torn tendons were not performed in these studies, which would have been the most accurate and definitive diagnosis of tendon conditions. Most studies to date have reported that the histological findings in ruptured tendons showed more degeneration than in normal tendons [12,13]. Yet, the degree of tendon degeneration and its impact on the structural integrity of the repaired tendon has not been studied in depth.
The purpose of this study was to investigate factors associated with postoperative structural integrity, particularly focusing on the histological assessment of supraspinatus tendons harvested during surgery. We hypothesized that tendon degeneration assessed using a semi-quantitative grading scale would affect the postoperative structural integrity of rotator cuff tendons.
The Institutional Review Board of SMG-SNU Boramae Medical Center approved this retrospective study (No. 06-2009-101), and informed consent was obtained from all participants.
The inclusion criteria were patients with (1) full-thickness rotator cuff tears treated by arthroscopic rotator cuff repair, (2) rotator cuff tendon tissue samples harvested at the time of surgery, and (3) availability of either postoperative MRI or computed tomography arthrography (CTA) at 1 year after surgery. Between July 2009 and May 2011, 213 consecutive patients were arthroscopically treated for shoulder problems at our institution. Among them, 146 patients with full-thickness rotator cuff tears were arthroscopically repaired. The remaining 67 patients were excluded, and comprised 49 partial-thickness rotator cuff tears, 1 isolated subscapularis tears, 5 infections, 9 bursitis, and 3 calcific tendinitis. Another 19 patients were excluded from the study since they did not undergo postoperative MRI or CTA at 1-year follow-up. An additional four patients did not undergo tissue biopsy. Finally, 56 patients were included in this retrospective study. Patients were grouped according to the structural integrity of the repaired rotator cuff tendon. Patients with intact tendon integrity according to 1-year follow-up MRI or CTA were classified as the healed group, and patients that showed failure in structural integrity were classified as the retear group.
All arthroscopic surgeries were performed by the same surgeon with patients in the lateral decubitus position under general anesthesia as previously described [2]. Briefly, systemic glenohumeral joint and subacromial exploration were performed, and lesions were managed as necessary. In each case, the rotator cuff tear was carefully evaluated after removing the frayed and atrophied torn end. Anteroposterior (AP) tear size, mediolateral (ML) retraction, number of involved tendons, excursion, presence of subscapularis tear, and gross tendon grade were documented [1,2]. Rotator cuff quality was evaluated using three grades based on gross appearance of the tendon at the time of surgery, based on three criteria: (1) fraying of over half the tendon thickness, (2) delamination, and (3) thinning to less than half the thickness of a normal rotator cuff, that is, less than 6 mm. Each tendon's overall quality was graded as A if none of these criteria were met, B if fraying or delamination were present, and C if both fraying and delamination or thinning were present. If footprint restoration was inadequate, tendon mobilization procedures including superior capsulotomy, coracohumeral ligament release, and medialization of the supraspinatus insertion in the greater tuberosity were performed to improve tendon excursion. Anterior or posterior interval slide was not performed in any patient. The footprint of the greater tuberosity was debrided using a shaver and Arthrocare, and only the unmineralized fibrocartilage layer of the cortical bone was removed. Debridement of bursal tissue, subacromial and distal clavicle osteophytes was minimally performed. Extensive acromioplasty to flatten a hooked or curved acromion was rarely performed. Tissue samples of 3×3 mm in size were harvested from the lateral edge of the torn supraspinatus tendon using a basket forceps after gentle shaving of the torn end. Rotator cuff repair was performed to cover the original footprint using the suture bridge technique whenever possible [1]. Suture anchors were inserted through the accessory portal. Generally, three to five suture anchors were used; one or two anchors for the medial row, and two or three anchors for the lateral row. Medial sutures were placed using either a 5.5-mm Healicoil anchor (Smith & Nephew) or a 5.5-mm Healix anchor (DePuy Synthes Mitek), and lateral sutures were placed using a either Quattro Link knotless anchor (Zimmer Biomet) or Footprint Ultra PK anchor (Smith & Nephew). All knots were tied securely using slippage-proof knots [14].
The shoulder was immobilized for 4 to 6 weeks using an abduction brace (4 weeks for small, medium, and large-sized tears, 6 weeks for massive tears). Shrugging, protraction, retraction of shoulder girdles, intermittent exercise of the elbow, wrist and hand, and external rotation of the arm to neutral with the brace were encouraged as tolerated, usually immediately after surgery. Further passive ROM and active-assisted ROM exercises were allowed after gradual weaning off of the abduction brace from 4 to 6 weeks after surgery. Patients began strengthening exercises after 3 months postoperatively. Light sports activities, such as jogging, were allowed after 3 months, and full return to sports was allowed at 6 to 9 months according to individual recovery.
Healed and retear groups were compared with respect to demographic, histological, clinical, structural, and arthroscopic factors that are thought to be associated with structural integrity of the repaired tendon. For histological assessment, harvested tendon specimens were fixed with 5 mL of neutral buffered 10% formalin in a plastic pathology container. Then, they were embedded in paraffin, cut into 4-µm thick sections, and stained with hematoxylin and eosin. Three sections were prepared per tendon and one of these was randomly selected and examined under a light microscope. Each slide was numbered using randomly generated numbers. After one of the investigators interpreted all of the slides once, the slides were arbitrarily renumbered using a new series of randomly generated numbers.
Tendon samples were graded using a modified semi-quantitative grading scale that assesses degeneration of the rotator cuff tendon [15,16]. The seven parameters included in the grading scale are: (1) fiber structure, (2) fiber arrangement, (3) rounding of the nuclei, (4) regional variations in cellularity, (5) increased vascularity, (6) decreased collagen stainability, and (7) hyalinization. A 4-point scoring system was used; 0 indicates normal; 1, slightly abnormal; 2, moderately abnormal; and 3, markedly abnormal. Overall, the total degeneration score for a given slide could vary from 0 (normal tendon) to 21 (most abnormal appearance detectable). All examinations were performed by two blinded investigators and two fellowship trained orthopedic surgeons. For each slide, the entire area was examined and the worst area was selected for comparisons between slides. The results were assessed for intra-observer and inter-observer reliability by reassessing the slides 1 week after the first examination. After assessing inter- and intra-observer variation, we used the results of the second examinations in this study.
Preoperative clinical outcomes were evaluated by the same orthopedic team. Prior to surgery, each patient completed a questionnaire that included standardized outcome assessments. Clinical factors included visual analog scale (VAS) pain score, ROM, strength, and preoperative functional scores (American Shoulder and Elbow Surgeons system, the Constant system, University of California Los Angeles system, Disabilities of Arm, Shoulder and Hand system, Simple Shoulder Test, and Shoulder Pain And Disability Index system). Clinical factors were assessed according to (1) pain, (2) ROM, (3) muscle strength, and (4) functional scores. VAS pain score was used to assess pain at rest, on motion and at night. Patients were asked to use a 10-cm scale marked from “no pain” to “unbearable pain.” The average VAS pain scores were calculated in both groups and compared preoperatively. ROM was measured with a goniometer in active forward flexion, abduction, external rotation with the arm at the side, and internal rotation. Internal rotation was measured using vertebral levels, and these were translated into numbers from 1 for the buttocks to 17 for T2 level. Flexion motor power, external rotation motor power, and internal rotation motor power were measured using a hand-held electronic scale (CHS; CAS).
To evaluate structural integrity, MRI (Achieva 3.0-T; Philips Medical System) with a dedicated shoulder coil or CTA (Ingenuity CT scanner; Philips) were performed at a minimum of 9 months after surgery. The structural integrity was evaluated using Sugaya’s classification for patients with MRI [17], or a modified Boileau’s grading system for patients with CTA [2,8]. In Sugaya’s classification, type I, II, and III were considered structurally healed tendons and type IV and V were considered retears. In the modified Boileau’s grading system, healed and incompletely healed were considered “healed,” while retear and new tear were considered “retear” [2,8]. All images were reviewed by a radiologist and an orthopedic surgeon. When there were discrepancies in reading, the worse evaluation was recorded.
Other structural factors included fatty infiltration and muscle atrophy. For the qualitative assessment of fatty infiltration, the Goutallier classification modified by Fuchs was used [18]. The classification has five stages, ranging from stage 0 (normal muscle) to stage 4 (more fat than muscle): stage 0, normal muscle without fat; stage 1, some fatty streaks within the muscle; stage 2, less fat than muscle; stage 3, same amount of fat and muscle within the muscle; and stage 4, more fat than muscle within the muscle. Muscular atrophy of the supraspinatus muscle was qualitatively graded using a modified Tangent sign [18]. The tangent line was drawn between superior margin of the scapular spine and coracoid. The grading system has three stages: grade 1, supraspinatus muscle crossing over the tangent line; grade 2, supraspinatus muscle meets the tangent line; and grade 3, most superior portion of the supraspinatus muscle is under the tangent line. The occupation ratio (OR) described by Thormazeau et al. [19] was used for grading the quantitative measurement of muscular atrophy.
Arthroscopic factors included rotator cuff tendon tear size, tendon retraction length, and tendon morphology. The extent of tear size and retraction was determined during operation under direct arthroscopic visualization after minimal debridement of the torn tendon edge. Macroscopic tendon quality was assessed based on three criteria: (1) fraying of over half the tendon thickness, (2) delamination, and (3) thinning to less than half the thickness of the normal rotator cuff, that is less than 6 mm. Gross tendon quality was graded as A if none of these criteria were met, B if either fraying or delamination was identified, and C if both fraying and delamination or thinning with or without the other two criteria were identified [2].
All analyses were performed using SPSS ver. 13.0 (SPSS Inc.). The mean values were compared using Student t-test or the Mann-Whitney U-test for continuous variables (age, symptom duration, symptom aggravation, total degeneration score, clinical scores, and tear size), and Pearson’s chi-square test or Fisher’s exact test for categorical variables (sex, fatty infiltration, tangent, occupation, tendon grading, and histological factors) to determine differences according to postoperative structural integrity. The paired t-test or Wilcoxon signed-rank test was used to compare preoperative functional scores. To validate tendon histopathology grading in this study, we assessed intra-observer and inter-observer variation by interclass correlation coefficient (ICC). Kappa statics were used to assess agreement between slide scores. In addition, multivariate logistic regression analysis was used to identify independent variables affecting outcomes using the stepwise forward conditional method. A P-value of 0.05 was considered significant.
Between July 2009 and May 2011, 56 patients were included in this study and retear was found in 12 patients (21.4%). Thus, 44 patients were grouped as the healed group, and the other 12 patients were grouped as the retear group. There were no significant preoperative differences in baseline demographic factors including gender, symptom duration, aggravation and dominance between the two groups (Table 1).
Histological assessment with a modified semi-quantitative grading scale showed high inter- and intra-observer reliability (Table 2). The ICCs of the inter-observer and intra-observer reliability for the total degeneration score were 0.899 (P<0.001) and 0.777 (P=0.001), respectively.
Compared with specimens from a previous study [20], normal tendon fibers were closely packed and parallel to each other (Fig. 1). Increased waviness and separation of the fibers accompanied slight to moderate changes (Fig. 2). Markedly abnormal specimens showed loss of fiber structure (Fig. 3). The mean scores of fiber structure in the healed group and the retear group were 2.82±0.49 and 2.83±0.38 (P=0.921), respectively (Table 3). The median score for the healed group was 3, and for the retear group was also 3.
In the control tendons, the fibers were parallel to each other. In ruptured and tendinopathic samples, this parallel arrangement was lost and haphazard. The mean scores in the healed group and the retear group were 2.61±0.65 and 2.67±0.65 (P=0.802), respectively. The median for the healed group was 3, and for the retear group it was also 3.
Normally, the tenocyte nuclei are flattened and spindle-shaped, sometimes arranged in rows (Fig. 1). In ruptured and tendinopathic samples, the tenocytes first decreased in number and then, as the pathologic changes progressed, the nuclei became progressively rounded (Figs. 2 and 3). The mean scores in the healed group and the retear group were 2.75±0.48 and 2.83±0.38 (P=0.584), respectively. The median for the healed group was 3, and for the retear group it was also 3.
Each whole slide was assessed for areas of increased cellularity. The mean scores in the healed group and the retear group were 2.20±0.85 and 2.08±0.79 (P=0.655), respectively. The median for the healed group was 2, and for the retear group it was also 2.
Vascular bundles usually run parallel alongside the collagen fibers. The number of these vascular bundles increases with degeneration of the tendon. The mean scores in the healed group and the retear group were 1.14±1.12 and 1.17±1.19 (P=0.938), respectively. The median for the healed group was 1, and for the retear group it was also 1.
Normal collagen is a deep pink-red when hematoxylin and eosin stain is added (Fig. 1). However, in degenerated collagen, the section stainability is reduced and appears paler. The mean scores in the healed group and the retear group were 1.32±1.05 and 1.42±0.79 (P=0.761), respectively. The median for the healed group was 1, and for the retear group it was also 1.
Ruptured tendons showed evidence of hyalinization (Fig. 3). The mean scores in the healed and the retear group were 1.09±1.17 and 1.08±1.31 (P=0.984), respectively. The median for the healed group was 1, and for the retear group it was 0.5.
The mean scores in the healed and the retear group were 13.93±2.03 and 14.08±2.23 (P=0.960), respectively. No histological variables were significantly different.
Preoperatively measured mean VAS pain scores at rest were 3.54±2.37 and 5.16±2.16 for the healed group and the retear group, respectively (P=0.046). Preoperatively measured mean VAS pain score at motion, mean VAS pain score at night, and average mean VAS pain scores showed no significant differences between the healed and the retear group (Table 4).
Neither active nor passive forward flexion nor abduction were significantly different between the healed and retear groups (Table 4).
No significant difference was found for preoperatively measured strength of the supraspinatus (Table 4).
The preoperatively measured functional scores of the healed and retear groups did not differ significantly (Table 4).
Mean fatty infiltration of the supraspinatus in the healed and the retear groups were 1.81±0.92 and 2.41±0.99 (P=0.055), respectively. Mean fatty infiltration of the infraspinatus in the healed and the retear groups were 1.30±0.79 and 1.51±0.52 (P=0.404), respectively. Mean fatty infiltration of the teres minor in the healed and the retear groups were 0.79 ±0.70 and 0.75±0.62 (P=0.840), respectively. Mean values of modified tangent sign in the healed and the retear groups were 1.30±0.46 and 1.67±0.88 (P=0.052), respectively. Mean values of occupation ratio grade in the healed and the retear groups were 1.70±0.66 and 2.08±0.79 (P=0.100), respectively. Although there were no significant differences among the two groups, mean values of structural factors in the healed group were lower for all variables.
Mean tear size of AP dimension in the healed and the retear groups were 24.30±12.35 mm and 36.42±25.23 mm, respectively (P=0.026). Mean retraction sizes measured in ML were 16.02±7.58 mm and 22.33±13.36 mm (P=0.037), respectively. The mean values of tendon grade in the healed and the retear group were 1.82±0.582 and 2.08±0.79 (P=0.199), respectively.
In the univariate analysis, the retear group showed significant differences with higher preoperative VAS pain score at rest (P=0.046) and larger tear size in both AP (P=0.026) and ML (P=0.037) dimensions compared to the healed group. Multivariable analysis showed that larger AP tear size was associated with postoperative structural integrity. The odds of retear rose by 4.2% for every 1mm increase in AP tear size (Exp(B): 1.042; 95% confidence interval: 1.004–1.082; P=0.032). AP tear size assessed during surgery can be used to estimate retear rate using the following logistic regression: logit(p)=β1×(AP tear size)+β2×(pre-VAS at rest)+β3(ML tear size).
The most important findings of this study were: (1) Postoperative structural integrity was not significantly affected by tendon degeneration; (2) the preoperative VAS pain score at rest and ML retraction size were significantly associated with the retear rate; and (3) the odds of retear rose by 4.2% for every 1-mm increase in AP tear size. Thus, tendon degeneration, which is associated with rotator cuff disease progression, was not significantly associated with retear risk in this study. This could be because the histologically examined torn tendons were all full-thickness tears, which represent the end stages of degeneration, and might not display significant differences in tear sizes. In contrast, there was a significant association between arthroscopically measured AP tear size and postoperative structural integrity.
In this study, none of the seven parameters nor the total degeneration score were significantly different between the healed and the retear groups (P=0.960). According to several previous studies, progressive degeneration of the tendon at the tendon-to-bone junction is one of the leading intrinsic causes of rotator cuff tears [21,22]. A histological study by Matthews et al. [23] reported that degenerative changes became more pronounced as the tear size increased to large and massive rotator cuff tears. In our study, however, no significant differences in tendon degeneration were found between patients with different-sized full-thickness rotator cuff tears. In several studies using the same histological grading system as that used in this study, the average total degeneration score for full-thickness tears ranged from 12 to 15 [24,25], suggesting that a total degeneration score of 12 or higher may indicate that tendon degeneration has already reached the end stages. The results of this study are consistent with those of previous studies [20,24]. Because a full-thickness rotator cuff tear is the final stage in the rotator cuff tear spectrum, it is possible that any degenerative changes in the torn tendons may already have progressed to the end stage, which is why no significant differences of tendon degeneration were found between the healed and retear groups.
In this study, the preoperatively measured average VAS pain score at rest in the healed group was 3.54±2.37, whereas it was 5.16±2.16 in the retear group (P=0.046). Several studies reported that the degenerative stages of the tendon did not vary with age or the size of the tears but were associated with symptom duration. In our previous study, we also found that symptom duration was correlated with the degree of tendon degeneration, suggesting that earlier rotator cuff repairs, possibly within 6 months of symptoms onset, will have better prognoses [26]. Thus, assessing pain intensity at the initial visit is beneficial for preventing further degeneration of the tendon through early diagnosis of full-thickness rotator cuff tears.
Larger tear sizes are associated with higher retear rates following surgery [27]. Le et al. [28] reported that tear size showed a stronger correlation with retear than other variables such as age and tissue quality, and Galatz et al. [7] reported that large and massive-sized tears showed higher retear rates than small-sized tears. In contrast, Jeong et al. [29] found that the primary tear size was of no significance, but rather, muscular atrophy of the supraspinatus muscle and fatty infiltration of the infraspinatus muscle were significant factors. In our study, medium tear sizes were observed in the healed group, whereas large tear sizes were observed in the retear group (P=0.026). Moreover, a medial retraction of 16.02±7.59 mm was observed in the healed group, whereas a medial retraction of 22.33±13.36 mm was observed in the retear group (P=0.037). Several previous studies reported that the extent of retraction could be a good predictor of retearing [30]. In this study, larger AP tear size was the only risk factor associated with retear in multivariate analysis. Through intraoperative measurements of AP tear size, surgeons should therefore be able to identify patients who are at risk for retear during arthroscopic repair and may choose to additionally perform multiple-channeling or other biological augmentation methods, thus decreasing the incidence of retear following surgery.
Our study had several limitations. First, the histological grading method may not fully reflect the degree of tendon degeneration since it lacks a standard measurement method. Although several studies have used the semiquantitative grading scale that was used in this study [15,16], the qualitative nature of histological variables made it difficult to quantitatively measure the variables. Second, there were no evaluations of myxoid, lipoid, or calcific degeneration in this system, which might have affected the results. Third, limited sample size and the mixed use of MRI and CTA for the evaluation of postoperative structural integrity are additional limitations. Fourth, we could not accurately assess the starting point of the tendon tear and the time between tendon tear and surgery, which might have affected histological grading and tear size and thus, surgical outcomes. In this study, the postoperative structural integrity of the rotator cuff tendon was not affected by tendon degeneration, whereas the arthroscopically measured AP tear size of the rotator cuff tendon was an independent predictor of retear.
NOTES
Fig. 1.
Hematoxylin and eosin stain of a control supraspinatus tendon in a 65-year-old female patient. Note closely packed, lightly stained parallel bundles of collagen fibers that contain flattened nuclei of tenocytes. Rounded nuclei are shown (original magnification, ×400). Fiber structure, 0; fiber arrangement, 0; rounding of the nuclei, 1; regional variations in cellularity, 0; increased vascularity, 0; decreased collagen stainability, 0; hyalinization, 0.
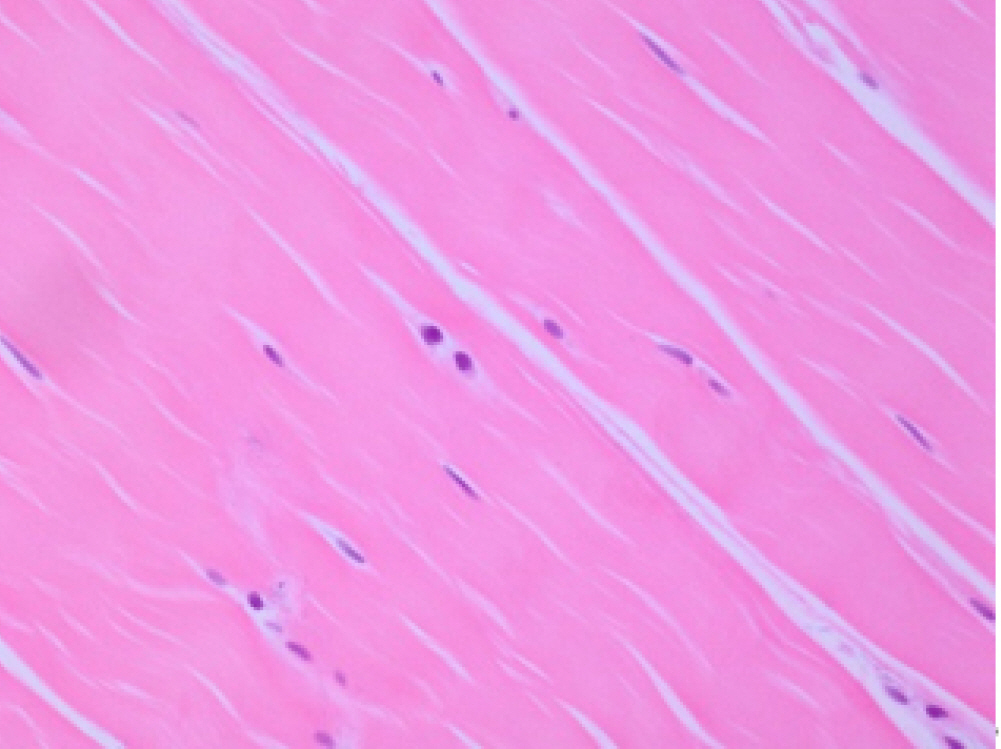
Fig. 2.
Hematoxylin and eosin stain of supraspinatus tendon harvested from ruptured supraspinatus tendon in a 61-year-old female. The collagen fibers have an undulating distribution (original magnification, ×200). Fiber structure, 3; fiber arrangement, 3; rounding of the nuclei, 3; regional variations in cellularity, 1; increased vascularity, 1; decreased collagen stainability, 0; hyalinization: 0.
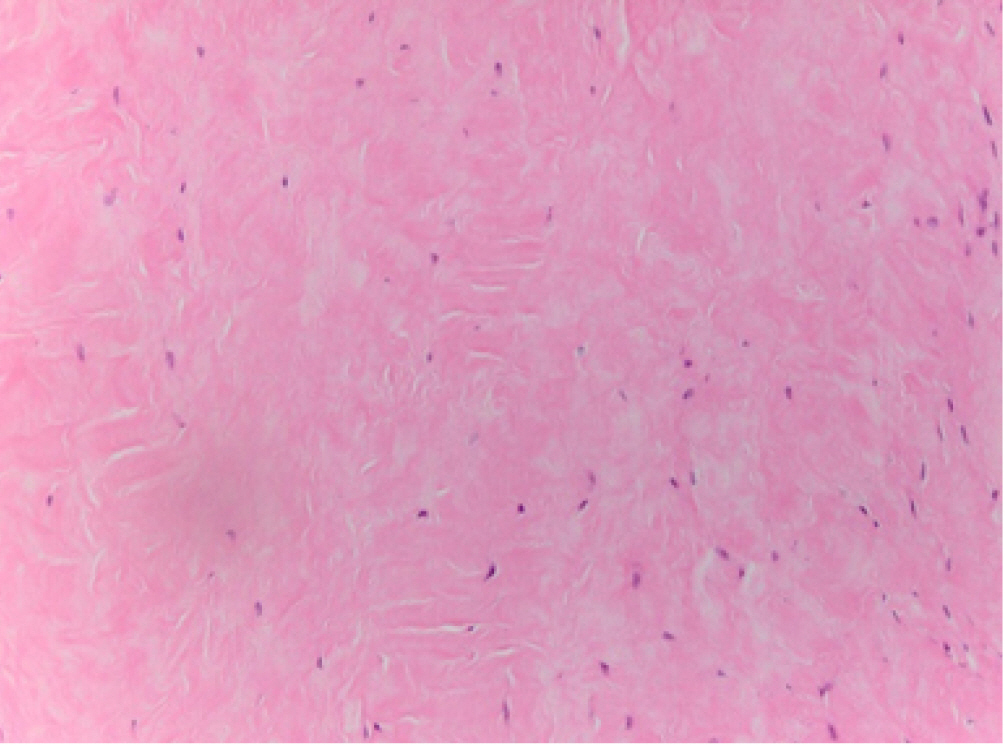
Fig. 3.
Hematoxylin and eosin stain of a control supraspinatus tendon in a 62-year-old female patient. Abnormal tenocytes resembling chondrocytes are shown (original magnification, ×400). Fiber structure, 3; fiber arrangement, 3; rounding of the nuclei, 3; regional variations in cellularity, 1; increased vascularity, 0; decreased collagen stainability, 2; hyalinization, 3.

Table 1.
Baseline demographics of patients
Table 2.
Inter-observer and intra-observer reliabilities
Table 3.
Comparison of histological parameters between the healed and the retear group
Table 4.
Comparison of preoperative variables between the healed and the retear group
| Category | Variable | Healed cuff (n=44) | Retear cuff (n=12) | P-value |
|---|---|---|---|---|
| Clinical | VAS at rest* | 3.54±2.37 | 5.16±2.16 | 0.046 |
| VAS at motion | 5.91±2.38 | 6.33±2.81 | 0.604 | |
| VAS at night | 5.44±2.75 | 6.91±2.10 | 0.092 | |
| VAS at average | 4.96±1.94 | 6.13±1.78 | 0.066 | |
| Flexion motor power (Ib) | 6.24±4.84 | 5.10±5.27 | 0.481 | |
| External rotation motor power (Ib) | 3.13±2.90 | 2.65±4.76 | 0.830 | |
| Internal rotation motor power (Ib) | 3.53±5.26 | 3.81±7.19 | 0.266 | |
| Active flexion ROM | 132.39±46.43 | 125.00±55.26 | 0.266 | |
| Active abduction ROM | 129.09±49.60 | 140±53.42 | 0.641 | |
| Passive flexion ROM | 147.39±31.88 | 145.83±51.02 | 0.897 | |
| Passive abduction ROM | 144.77±38.20 | 152.50±46.24 | 0.555 | |
| ASES system | 46.52±18.42 | 35.00±17.44 | 0.058 | |
| Constant system | 46.20±18.05 | 43.85±18.19 | 0.692 | |
| UCLA system | 15.11±5.44 | 13.16±4.46 | 0.261 | |
| DASH system | 48.62±21.54 | 59.16±22.67 | 0.144 | |
| SST | 4.40±2.93 | 3.91±3.36 | 0.625 | |
| SPADI | 54.50±21.24 | 63.94±23.29 | 0.188 | |
| Structural | Fatty infiltration of the supraspinatus | 1.81±0.92 | 2.41±0.99 | 0.055 |
| Fatty infiltration of the infraspinatus | 1.30±0.79 | 1.51±0.52 | 0.404 | |
| Fatty infiltration of the teres minor | 0.79±0.70 | 0.75±0.62 | 0.840 | |
| Modified tangent sign | 1.30±0.46 | 1.67±0.88 | 0.052 | |
| Occupation ratio grade | 1.70±0.66 | 2.08±0.79 | 0.100 | |
| Arthroscopic | Tear size (antero-posterior)* (mm) | 24.30±12.35 | 36.42±25.23 | 0.026 |
| Tear size (medio-lateral)* (mm) | 16.02±7.58 | 22.33±13.36 | 0.037 | |
| Tendon grading (A:B:C, mean score) | 1.82±0.58 (12:28:4) | 2.08±0.79 (3:5:4) | 0.199 |
Values are presented as mean±standard deviation.
VAS: visual analog scale, ROM: range of motion, ASES: American Shoulder and Elbow Surgeons, UCLA: University of California Los Angeles, DASH: Disabilities of Arm, Shoulder and Hand, SST: Simple Shoulder Test, SPADI: Shoulder Pain And Disability Index.
REFERENCES
1. Jo CH, Shin JS, Lee YG, et al. Platelet-rich plasma for arthroscopic repair of large to massive rotator cuff tears: a randomized, single-blind, parallel-group trial. Am J Sports Med 2013;41:2240–8.


2. Jo CH, Yoon KS, Lee JH, et al. The effect of multiple channeling on the structural integrity of repaired rotator cuff. Knee Surg Sports Traumatol Arthrosc 2011;19:2098–107.


3. Kinsella K, Velkoff VA. An ageing world. The US Census Bureau, US Government Printing Office; 2001.
4. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am 2012;94:227–33.



5. Duquin TR, Buyea C, Bisson LJ. Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am J Sports Med 2010;38:835–41.


6. Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg 2006;15:290–9.


7. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am 2004;86:219–24.


8. Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal. J Bone Joint Surg Am 2005;87:1229–40.


9. Nho SJ, Brown BS, Lyman S, Adler RS, Altchek DW, MacGillivray JD. Prospective analysis of arthroscopic rotator cuff repair: prognostic factors affecting clinical and ultrasound outcome. J Shoulder Elbow Surg 2009;18:13–20.


10. Keener JD, Wei AS, Kim HM, et al. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome. J Bone Joint Surg Am 2010;92:590–8.


11. Cho NS, Rhee YG. The factors affecting the clinical outcome and integrity of arthroscopically repaired rotator cuff tears of the shoulder. Clin Orthop Surg 2009;1:96–104.



12. Longo UG, Franceschi F, Ruzzini L, et al. Characteristics at haematoxylin and eosin staining of ruptures of the long head of the biceps tendon. Br J Sports Med 2009;43:603–7.


13. Maffulli N, Testa V, Capasso G, et al. Similar histopathological picture in males with Achilles and patellar tendinopathy. Med Sci Sports Exerc 2004;36:1470–5.


14. Jo CH, Lee JH, Kang SB, et al. Optimal configuration of arthroscopic sliding knots backed up with multiple half-hitches. Knee Surg Sports Traumatol Arthrosc 2008;16:787–93.


15. Cook JL, Feller JA, Bonar SF, Khan KM. Abnormal tenocyte morphology is more prevalent than collagen disruption in asymptomatic athletes' patellar tendons. J Orthop Res 2004;22:334–8.


16. Maffulli N, Barrass V, Ewen SW. Light microscopic histology of achilles tendon ruptures: a comparison with unruptured tendons. Am J Sports Med 2000;28:857–63.


17. Sugaya H, Maeda K, Matsuki K, Moriishi J. Functional and structural outcome after arthroscopic full-thickness rotator cuff repair: single-row versus dual-row fixation. Arthroscopy 2005;21:1307–16.


18. Fuchs B, Weishaupt D, Zanetti M, Hodler J, Gerber C. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg 1999;8:599–605.


19. Thomazeau H, Rolland Y, Lucas C, Duval JM, Langlais F. Atrophy of the supraspinatus belly: assessment by MRI in 55 patients with rotator cuff pathology. Acta Orthop Scand 1996;67:264–8.


20. Jo CH, Shin WH, Park JW, Shin JS, Kim JE. Degree of tendon degeneration and stage of rotator cuff disease. Knee Surg Sports Traumatol Arthrosc 2017;25:2100–8.


21. Tallon C, Maffulli N, Ewen SW. Ruptured Achilles tendons are significantly more degenerated than tendinopathic tendons. Med Sci Sports Exerc 2001;33:1983–90.


22. Kovacevic D, Rodeo SA. Biological augmentation of rotator cuff tendon repair. Clin Orthop Relat Res 2008;466:622–33.



23. Matthews TJ, Hand GC, Rees JL, Athanasou NA, Carr AJ. Pathology of the torn rotator cuff tendon: reduction in potential for repair as tear size increases. J Bone Joint Surg Br 2006;88:489–95.

24. Jo CH, Chang MS. Degeneration exists along the entire length of the supraspinatus tendon in patients with a rotator cuff tear. Clin Shoulder Elbow 2015;18:61–7.

25. Longo UG, Franceschi F, Ruzzini L, et al. Histopathology of the supraspinatus tendon in rotator cuff tears. Am J Sports Med 2008;36:533–8.


26. Jo CH, Lee YJ, Lee YG, Lee JM, Kim JE. Factors associated with degeneration of rotator cuff tendon: a histological study in patients with rotator cuff tear. Acta Orthop Belg 2019;85:393–9.

27. Choi S, Kim MK, Kim GM, Roh YH, Hwang IK, Kang H. Factors associated with clinical and structural outcomes after arthroscopic rotator cuff repair with a suture bridge technique in medium, large, and massive tears. J Shoulder Elbow Surg 2014;23:1675–81.


28. Le BT, Wu XL, Lam PH, Murrell GA. Factors predicting rotator cuff retears: an analysis of 1000 consecutive rotator cuff repairs. Am J Sports Med 2014;42:1134–42.










