When should reverse total shoulder arthroplasty be considered in glenohumeral joint arthritis?
Article information
Abstract
Anatomical total shoulder arthroplasty (TSA) has been used widely in treatment of glenohumeral osteoarthritis and provides excellent pain relief and functional results. Reverse total shoulder arthroplasty (RSA) was created to treat the complex problem of rotator cuff tear arthropathy. RSA also has been performed for glenohumeral osteoarthritis even in cases where the rotator cuff is preserved and has shown good results comparable with TSA. The indications for RSA are expanding to include tumors of the proximal humerus, revision of hemiarthroplasty to RSA, and revision of failed TSA to RSA. The purposes of this article were to describe comprehensively the conditions under which RSA should be considered in glenohumeral osteoarthritis, to explain its theoretical background, and to review the literature.
INTRODUCTION
Anatomical total shoulder arthroplasty (TSA) has been used widely in treatment of glenohumeral osteoarthritis (GHOA) and provides excellent pain relief and functional results [1-3]. As TSA is designed for restoring the biomechanics of a normal shoulder joint, adequate glenoid bone stock and intact rotator cuff tendons are essential for good results. Biomechanically, the TSA needs soft tissue balance and must permit translation in the glenohumeral joint. Reverse total shoulder arthroplasty (RSA) was created to treat the complex problem of rotator cuff tear arthropathy [4]. Biomechanically, the RSA provides a stable and fixed fulcrum of the arm for rotation, while increasing the moment arm and resting tension of the deltoid muscle, which enable arm elevation and abduction, even in massive rotator cuff tears [5,6]. For the last three decades, RSA for cuff tear arthropathy has been successful. RSA can be used not only for patients with cuff tear arthropathy, but also for those with other complex shoulder problems in whom the soft tissues or glenoid bone stock can be deficient. The indications for its use are expanding to include tumors of the proximal humerus, revision of hemiarthroplasty to RSA, and revision of failed TSA to RSA [4,6-8].
TSA requires restoration of the normal shoulder construct in soft tissue balance and bony architecture. If preoperative factors related with poor clinical outcomes in TSA are uncorrectable, satisfactory results cannot be obtained. In this situation, RSA could be an alternative option. Upon literature review, three factors have been mentioned commonly as related with poor clinical outcomes: rotator cuff dysfunction, glenoid bone deformity, and preoperative stiffness. These three factors independently can influence the outcome of TSA but sometimes coexist and can influence each other [9,10].
In terms of soft tissue balance, rotator cuff condition is the most important factor. Postoperative rotator cuff tear can cause instability, which can progress to glenoid loosening and failure. Preoperative rotator cuff tear, rotator cuff muscle atrophy (MA), and fatty infiltration (FI) are correlated with poor clinical results [1,9,11-13]. In terms of bony architecture, uncorrectable glenoid deformities (e.g., glenoid retroversion, posterior erosion, and humeral head subluxation) are negative factors related with poor clinical results after TSA. Joint stiffness is another negative factor commonly comorbid in TSA. The appropriate treatment of patients with GHOA and significant stiffness (limitation of motion) remains a controversial clinical dilemma [1,14]. Stiff shoulders can be associated with significant rotator cuff muscle dysfunction, even in the absence of a full-thickness rotator cuff tear. Advanced age and long-standing stiffness have been linked to increased FI and MA [10,13].
The goals of this study are (1) to describe the three conditions (rotator cuff dysfunction, glenoid bone deformity, and stiffness) in which RSA should be considered for treatment of GHOA, (2) to review the clinical and mechanical background of RSA and TSA, and (3) to review published clinical outcomes of RSA for treatment of GHOA.
ROTATOR CUFF TEAR
Clinical Outcome of RSA in GHOA with Intact Cuff
Recently, RSA has been performed for GHOA even in cases where the rotator cuff is preserved and has shown good results comparable with those of TSA. Wright et al. [14] compared TSA and RSA in patients 70 years and older with GHOA and an intact rotator cuff. There was no difference in patient-reported outcome measures, range of motion, American Shoulder and Elbow Surgeons (ASES) score, Western Ontario Osteoarthritis of the Shoulder index, or complication rate or revision surgery rate between the groups. All patients of the RSA group and 98% of the TSA group could achieve full or nearly full (>135°) forward elevation at a minimum of two years after the procedure [14]. Steen et al. [15] evaluated 24 consecutive GHOA patients who underwent RSA and matched them to 96 patients who underwent TSA. Postoperative ASES, Simple Shoulder Test score, and range of motion were similar between the groups. There was no significant difference in complication rate or revision surgery rate between groups. However, five TSA patients showed radiographic glenoid loosening, whereas no RSA patients did [15].
Incidence of rotator cuff tear in GHOA
The incidence of rotator cuff tear in the asymptomatic elderly population is high. Khoschnau et al. [16] evaluated prevalence of rotator cuff tears in a population with a mean age of 66 years who had never sought care for shoulder symptoms. Of the 106 individuals (212 shoulders), the prevalence of full-thickness cuff tear was 30% (21% of 212 shoulders). Another study investigated the clinical and ultrasonography results of shoulders from 420 asymptomatic volunteers aged between 50 and 79 years. Full-thickness tear of the rotator cuff was detected in 32 individuals (7.6%). The prevalence increased with age as follows: 50 to 59 years, 2.1%; 60 to 69 years, 5.7%; and 70 to 79 years, 15% [17]. Minagawa et al. [18] evaluated 664 residents in one village who had undergone ultrasonography. The prevalence of rotator cuff tear in the general population was 22.1%, which increased with age. Asymptomatic tear was twice as common as symptomatic tear. However, the incidence of rotator cuff tear in GHOA patients is controversial. Edwards et al. [13] described the results of TSA in 555 osteoarthritic shoulders, of which 42 (7.6%) had a rotator cuff tear. In Iannotti and Norris's study [1], most (n=115; 90%) of 128 shoulders had a structurally intact rotator cuff. Thirteen were found to have a full thickness tear, but only seven (5% of 128) had a tear >1 cm. However, since patients with large rotator cuff tear might be excluded from TSA study, the incidence of rotator cuff tear could be underestimated.
Significancy of rotator cuff in TSA
In GHOA, rotator cuff conditions are variable in tear size, cuff thickness, MA, and FI. Sometimes, even without cuff tear, MA and FI can be severe, or rotator cuff tendon can be thin. Several classifications have been used to describe the condition of rotator cuff in terms of MA, FI, and tear size [19-21]. GHOA commonly is accompanied by degenerative changes in the rotator cuff [22]. However, none of the classifications adequately describe the degenerative degree of the rotator cuff.
The size of the rotator cuff tear before surgery should be considered carefully. A repairable tear of the supraspinatus tendon is not a contraindication to TSA. If partial tear or small rotator cuff tears are well repaired during TSA surgery, they have little influence on the results of shoulder arthroplasty [1]. Raval et al. [23] evaluated 36 patients with a mean age 79.2 years who underwent TSA and had GHOA with partial-thickness rotator cuff tears observed on MRI for a mean follow-up of 5.8 years. The study showed that presence of a partial cuff tear on preoperative MRI does not significantly affect function after anatomical TSA in the medium-term follow-up. However, a medium- to large-sized full thickness rotator cuff tear negatively influences the results of shoulder arthroplasty. Simone et al. [2] evaluated 33 patients who had rotator cuff repair with TSA for a mean follow-up of 4.7 years. Instability and glenoid loosening occurred in six patients with medium or large tear. Complications were noted in five patients, all with medium or large tear; four of these had symptomatic instability and one sustained a late peri-prosthetic fracture. Four patients required further surgery, three due to instability and one due to peri-prosthetic humeral fracture [2]. Coexistent tears of the rotator cuff prejudice the outcome of TSA by reducing active movement and strength and by predisposing to instability or subluxation of the replacement and loosening of the glenoid component (Fig. 1). Postoperative rotator cuff tears in TSA are not only related with decreased range of motion, but also instability or subluxation, which eventually lead to early glenoid loosening [1,12,13,24].
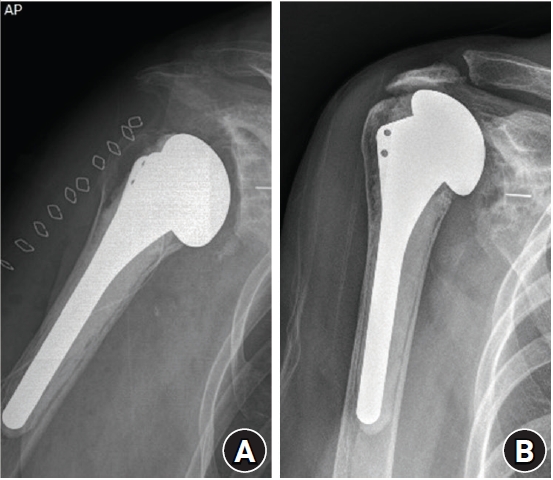
(A) Immediate postoperative radiograph after total shoulder arthroplasty shows normal glenohumeral distance and contiguous scapulohumeral line. (B) In 3-year follow-up radiograph, superior migration (decreased acromiohumeral distance) and osteolysis around glenoid component are observed from the postoperative rotator cuff tear.
Subscapularis tear after TSA is a common complication but cannot be diagnosed reliably by physical examination or radiographs. Although there is an opinion that subscapularis integrity does not correlate with pain or subjective patient outcome, inadequate healing of the subscapularis tendon can lead to postoperative pain, weakness, and instability [25-27]. Postoperative subscapularis tear could induce upward migration of the humeral head, anterosuperior subluxation, an eccentric contact pattern, and higher stress to the glenoid component [28].
The clinical significance of subscapularis repair is controversial in RSA. A prospective randomized trial by Engel et al. [29] concluded that subscapularis tendon repair in RSA improves the Constant score and internal rotation at 12 months after surgery. In medialized design RSA, the subscapularis has an important role in preventing dislocation [30]. Although subscapularis repair is safe and effective for RSA, it cannot offer additional clinical or functional benefit in patients treated with lateralized RSA [31]. Therefore, in GHOA with inadequate subscapularis condition where postoperative retear is expected, RSA could be considered.
In addition to rotator cuff tear, MA and FA should be considered preoperatively in TSA. In GHOA patients, FI and MA of the rotator cuff are major factors associated with clinical outcomes after TSA. Conversely, they are not significant in RSA. Puzzitiello et al. [12] concluded that rotator cuff muscle quality as assessed by MA and FI does not impact clinical outcomes following RSA with a lateralized glenosphere in patients with GHOA and an intact rotator cuff. Therefore, if progressed MA and FI is combined with GHOA, RSA could be a reasonable decision even with an intact rotator cuff [2,10,12,13].
GLENOID DEFORMITY (GLENOID BONE LOSS, POSTERIOR GLENOID WEAR, AND INCREASED RETROVERSION)
Normal anatomy of glenoid
Prosthetic design and surgical considerations related to glenoid anatomy are based on numerous studies focusing on glenoid height, width, inclination, and version [32]. The ‘‘normal’’ range of glenoid version varies anywhere from 2° of anteversion to 8° of retroversion in most studies [33-35]. Studies using three-dimensional measurement techniques on computed tomography (CT) images have reported native glenoid version of approximately 7° [36,37]. However, arthritic shoulders generally have greater than 11° of retroversion, which should be corrected during TSA [33,38,39].
Classification and clinical significance of glenoid deformity
Walch et al. [40,41] devised a classification system for glenoid morphology that is based on the architecture and patterns of posterior wear in GHOA [32]. In type B2 glenoids, posterior humeral head subluxation and posterior glenoid wear can increase glenoid retroversion to values above 10°. Type C glenoids with evidence of dysplasia can show glenoid retroversion above 25°. Failure to replicate and restore characteristics of the normal glenoid articular surface can lead to early loosening. This can be particularly difficult in patients with biconcave glenoids and associated posterior humeral head instability. Failure to restore neutral glenoid version can increase the shear load across the glenoid. This subtype has problems in soft tissue balancing and is associated with a high rate of revision surgery because of glenoid loosening and instability [11,32,41,42].
Posterior wear and increased retroversion in glenohumeral arthritis often is associated with static posterior subluxation of the humeral head. Static posterior subluxation could be reversed by TSA using corrective glenoid reaming and soft tissue release [43]. However, if static posterior subluxation persists after TSA, postoperative subluxation can lead to eccentric loading of the glenoid component and accelerated loosening and wear [11,32,41,43].
Variable methods of correcting version and increasing stability of the glenoid component have been used intraoperatively. Asymmetric glenoid reaming, posterior glenoid bone grafting, and use of specialized glenoid implants are included. The choice among these options is based on the ability to assess accurately both glenoid version and the desired amount of correction intraoperatively. However, this often is limited by obscured bony landmarks and deficient bone stock. Even with preoperative three-dimensional imaging, implantation of a glenoid component to within 10° of a desired version is technically difficult even for an experienced shoulder surgeon in cases of severe retroversion [44].
Bone Grafting in TSA and RSA
Bone graft could be used in TSA in patients with osteoarthritis combined with increased retroversion or biconcavity. Bone grafting with internal fixation is a reconstructive technique with mixed clinical results. It has been used in limited cases of large segmental bone deficiencies or in cases of severe posterior wear causing severe component loosening. However, a very high rate of complications after bone graft such as bone resorption, non-union, and early loosening were found. Because of the high complication rate of posterior bone graft with an anatomic prosthesis, Walch et al. [41] recommend RSA instead of TSA with neoglenoid retroversion [11,32,44]. However, the bone healing rate is very high and predictable in RSA [45].
DIFFERENCES IN BONE GRAFT BETWEEN TSA AND RSA
RSA has some advantages compared to TSA. (1) Stable fixation: variable angle locking screw fixation creates a more stable construct and reduces baseplate micromotion. However, in TSA, cement-type fixation for glenoid components has been used commonly and can interfere with bone union. Bone grafting with internal fixation is a technically demanding procedure in TSA. The period of motion restriction can be prolonged, and the success rate is not high [32]. (2) Diminished force to graft: unequal radii of curvature between the humeral and glenoid components of TSA permit translation movement, which produces shearing force between the glenoid component and bone graft. However, a reverse prosthesis, designed with equal radii of curvature, can tolerate a joint-reaction force vector. Increased constraint secondary to the deeper and greater conformity of the concavity of the humeral articular surface prevents glenohumeral translation while providing sufficient stability [8,46]. (3) As the burden of restoring the joint line and soft tissue balance is lower in RSA than TSA, glenoid bone graft could be thick enough to achieve firm fixation. (4) It is easier to correct glenoid version in RSA than TSA as an asymmetric bone graft is possible [47-49].
STIFFNESS
Joint stiffness can cause difficulties in any surgery performed on the shoulder joint. In TSA, preoperative stiffness corresponds to a major risk of poor clinical results, and recovery of range of motion is difficult even after surgery. Stiffness is the most common cause of failure in TSA and postoperative stiffness has been considered a type of failure [9]. Joint stiffness can be confirmed by a decrease in passive range of motion [1].
The difficulties encountered during TSA procedure in a stiff shoulder joint are as follows. (1) Joint stiffness deteriorates the operation field exposure for the glenoid procedure, which is one of the reasons for difficult operation. (2) If the glenoid is not exposed sufficiently, problems can occur during glenoid preparation and glenoid component positioning. This condition could increase the risk of glenoid malposition, which leads to early loosening of the glenoid implant [50]. (3) Nerve injury is very rare in shoulder arthroplasty. However, in shoulders with stiffness, the risk of nerve injury could increase as excessive soft tissue traction or soft tissue release often is required [51-53]. (4) It often is difficult to repair the rotator cuff after implantation. The rotator cuff of a shoulder with longstanding stiffness could be irreparable after implantation or have short tendon excursion. In this condition, repair of the subscapularis can be incomplete or not possible, which could increase the extent of future subscapularis tear [3,9].
In RSA on a stiff shoulder, there are many difficulties. As the glenoid component of RSA is larger than that of TSA, a larger exposure is required in RSA. In addition, the risk of fracture could increase during the reduction step of the procedure. Due to the convexity of the glenoid component, reduction requires sufficient traction. During this step, greater tuberosity fracture could occur. As the humerus is retracted posteriorly using a traction tool to expose the glenoid in the stiff shoulder joint, bone erosion or fracture by the traction tool can occur in the humeral head [54].
Despite the above difficulties, RSA is preferred for GHOA with stiffness because the results of TSA are inferior. Though there is no study comparing TSA and RSA in GHOA with stiffness, RSA could result in potentially good results even in stiff shoulders [54]. There are several technical advantages of RSA in GHOA with stiffness. (1) Freedom from rotator cuff preservation: in TSA, the rotator cuff tendon should be preserved and repaired at the last stage of surgery. In addition, MA and FI are important [10]. However, in RSA, the tendons of supraspinatus and infraspinatus can be removed, and MA and FI are not critical to clinical outcomes [12]. Moreover, superior rotator cuff removal could improve surgical field exposure and allows soft tissue release [12,31]. (2) Greater capacity of humeral bone cutting: in RSA compared to TSA, greater humeral head cutting is possible beyond the rotator cuff attachment. Increased humeral bone cutting improves the surgical field exposure and stiffness. (3) Freedom from subscapularis repair: as described in the previous paragraph, lateralized implantation of RSA does not require subscapularis repair. This is helpful to reduce soft tissue tension during operation and to prevent postoperative stiffness [31,55].
CONCLUSIONS
The indications for RSA gradually are expanding. For GHOA with intact rotator cuff, TSA is the gold standard treatment. However, RSA could be adopted. It is reasonable that RSA is selected preferentially for treatment of GHOA in which specific conditions are combined, such as rotator cuff degeneration (greater than 1 cm tear, advanced MA or FI, subscapularis insufficiency), glenoid bone deformity (glenoid bone loss or retroversion needing bone graft), and stiffness.
Notes
Financial support
None.
Conflict of interest
None.
